In Vitro Diagnostic Device Market
In Vitro Diagnostic Device Market Size, Scope, Growth, Trends and By Segmentation Types, Applications, Regional Analysis and Industry Forecast (2025-2033)
Report ID : RI_703856 | Last Updated : August 05, 2025 |
Format : ![]()
![]()
![]()
![]()
In Vitro Diagnostic Device Market Size
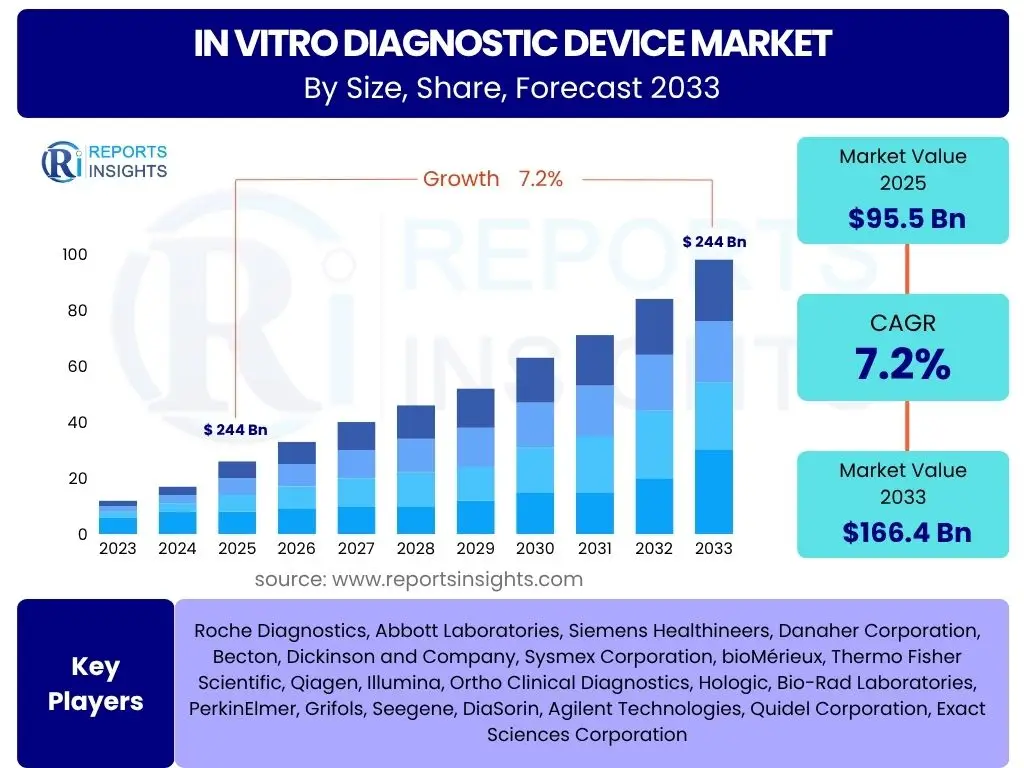
According to Reports Insights Consulting Pvt Ltd, The In Vitro Diagnostic Device Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 7.2% between 2025 and 2033. The market is estimated at USD 95.5 Billion in 2025 and is projected to reach USD 166.4 Billion by the end of the forecast period in 2033.
Key In Vitro Diagnostic Device Market Trends & Insights
The In Vitro Diagnostic Device market is experiencing transformative shifts driven by technological innovation and evolving healthcare demands. Key trends revolve around the accelerating adoption of molecular diagnostics, particularly in oncology and infectious disease testing, alongside a marked shift towards decentralized, point-of-care testing solutions. Furthermore, the integration of digital health platforms and artificial intelligence is enhancing diagnostic accuracy and efficiency, supporting a move towards personalized medicine. These trends collectively underscore a market moving towards more accessible, rapid, and precise diagnostic capabilities.
- Growing emphasis on point-of-care testing (POCT) for rapid and accessible diagnostics.
- Rising adoption of molecular diagnostics for infectious diseases and genetic testing.
- Increasing integration of automation and robotics in laboratory workflows.
- Development of companion diagnostics for targeted therapies and personalized medicine.
- Expansion of digital pathology and telehealth platforms enabling remote diagnostics.
AI Impact Analysis on In Vitro Diagnostic Device
Artificial Intelligence is poised to revolutionize the In Vitro Diagnostic Device sector by enhancing diagnostic precision, streamlining laboratory operations, and enabling more sophisticated data analysis. Users are increasingly seeking to understand how AI can improve the speed and accuracy of disease detection, automate complex testing procedures, and provide predictive insights for patient care. Expectations include AI's role in interpreting large volumes of diagnostic data, reducing human error, and facilitating the development of novel diagnostic algorithms, ultimately leading to more personalized and effective treatment strategies. AI's influence extends to every phase, from research and development to clinical application, optimizing resource allocation and improving overall healthcare outcomes.
- Enhanced diagnostic accuracy through advanced image analysis and pattern recognition.
- Automation of routine laboratory tasks, reducing manual errors and improving throughput.
- Predictive analytics for disease progression and personalized treatment response.
- Streamlined data interpretation from complex diagnostic tests, such as genomics.
- Development of intelligent diagnostic algorithms for early disease detection.
Key Takeaways In Vitro Diagnostic Device Market Size & Forecast
The In Vitro Diagnostic Device market is poised for robust and sustained growth, driven by escalating global health challenges and continuous technological innovation. Key takeaways highlight a significant increase in market valuation, fueled by the rising prevalence of chronic and infectious diseases, a growing aging population, and the expansion of healthcare infrastructure in emerging economies. The forecast indicates a strong trajectory, underscoring the critical role of IVD devices in proactive disease management, personalized medicine, and overall public health initiatives. This growth reflects the ongoing investment in research and development aimed at creating more efficient, accurate, and accessible diagnostic tools worldwide.
- The market is projected for substantial growth, driven by increasing disease burden and technological advancements.
- Significant expansion in emerging economies, fueled by improved healthcare access and awareness.
- Continued innovation in molecular diagnostics and personalized medicine will be key growth enablers.
- Demand for rapid and accurate diagnostic tools will remain high across various healthcare settings.
- The sector demonstrates resilience and adaptability to evolving global health crises and clinical needs.
In Vitro Diagnostic Device Market Drivers Analysis
The In Vitro Diagnostic Device market is significantly propelled by several synergistic factors. A primary driver is the escalating global incidence of chronic and infectious diseases, necessitating widespread and accurate diagnostic testing for effective disease management and control. Concurrently, the increasing elderly population, a demographic more susceptible to various health conditions, further fuels the demand for comprehensive diagnostic solutions. These demographic shifts, combined with continuous advancements in diagnostic technologies and a growing emphasis on preventive healthcare, create a robust environment for market expansion, driving innovation and adoption across healthcare systems worldwide.
| Drivers | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Rising prevalence of chronic and infectious diseases | +2.5% | Global, particularly Asia Pacific & Africa | 2025-2033 |
| Technological advancements in diagnostic techniques | +2.0% | North America, Europe, Japan | 2025-2033 |
| Growing geriatric population and associated health issues | +1.5% | Europe, North America, Japan, China | 2025-2033 |
| Increasing demand for personalized medicine | +0.8% | North America, Western Europe | 2027-2033 |
| Expanding healthcare infrastructure in developing economies | +0.4% | Asia Pacific, Latin America | 2025-2033 |
In Vitro Diagnostic Device Market Restraints Analysis
Despite its significant growth potential, the In Vitro Diagnostic Device market faces several notable restraints that could temper its expansion. The high cost associated with advanced IVD tests and sophisticated instrumentation presents a substantial barrier, particularly in price-sensitive markets and for individuals with limited insurance coverage. Furthermore, stringent regulatory approval processes, varying widely across different regions, can prolong market entry for new products and increase development costs. These factors, alongside potential reimbursement challenges and the need for specialized infrastructure, collectively pose hurdles to the widespread adoption and accessibility of innovative diagnostic solutions.
| Restraints | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| High cost of IVD tests and instruments | -1.2% | Global, particularly emerging markets | 2025-2033 |
| Stringent regulatory frameworks and approval processes | -0.9% | North America, Europe | 2025-2030 |
| Reimbursement challenges and policies | -0.7% | North America, Europe | 2025-2033 |
| Lack of skilled professionals for advanced IVD technologies | -0.5% | Developing regions, remote areas | 2025-2033 |
In Vitro Diagnostic Device Market Opportunities Analysis
The In Vitro Diagnostic Device market is abundant with promising opportunities, particularly driven by unmet medical needs and technological convergence. The burgeoning demand for point-of-care diagnostics presents a significant avenue for growth, offering rapid results and improved patient access, especially in remote or resource-limited settings. Moreover, the increasing focus on companion diagnostics, which link specific tests to targeted therapies, opens new markets within personalized medicine. The expansion into emerging economies, coupled with the integration of cutting-edge technologies like artificial intelligence and machine learning, further amplifies the potential for market innovation and expansion, providing pathways for diverse new applications and enhanced diagnostic capabilities.
| Opportunities | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Development and adoption of point-of-care diagnostics (POCT) | +1.8% | Global, particularly rural areas and emergency settings | 2025-2033 |
| Emergence of companion diagnostics for targeted therapies | +1.5% | North America, Europe, Developed Asia Pacific | 2026-2033 |
| Growth in emerging economies with improving healthcare infrastructure | +1.3% | Asia Pacific, Latin America, Middle East | 2025-2033 |
| Integration of AI, machine learning, and big data analytics | +1.0% | Global, particularly tech-forward regions | 2027-2033 |
| Increasing focus on home-based testing and remote monitoring | +0.7% | North America, Europe, Oceania | 2025-2033 |
In Vitro Diagnostic Device Market Challenges Impact Analysis
The In Vitro Diagnostic Device market encounters various challenges that can impact its growth and widespread adoption. One significant hurdle is the continuous threat of rapid technological obsolescence, necessitating constant research and development investments to stay competitive. Data privacy and security concerns, especially with the increasing digital integration of diagnostic results and patient data, present complex regulatory and ethical challenges. Furthermore, disruptions in the global supply chain, as witnessed recently, can severely impact the availability of essential reagents and instruments. These challenges require robust strategies, including flexible manufacturing, strong cybersecurity measures, and adaptable regulatory compliance, to mitigate their potential adverse effects on market stability and growth.
| Challenges | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Rapid technological obsolescence | -0.8% | Global | 2025-2033 |
| Data privacy and security concerns | -0.6% | North America, Europe | 2025-2033 |
| Supply chain disruptions and raw material shortages | -0.5% | Global | 2025-2028 |
| Ethical considerations for genetic and personalized testing | -0.3% | Global, particularly sensitive markets | 2025-2033 |
In Vitro Diagnostic Device Market - Updated Report Scope
This report provides a comprehensive analysis of the In Vitro Diagnostic Device market, encompassing historical data from 2019 to 2023, current market estimations for 2025, and future projections up to 2033. It offers in-depth insights into market size, growth rates, key trends, and the impact of technological advancements such as AI. The scope includes detailed segmentation by product, technology, application, and end-user, along with regional analyses to provide a holistic view of market dynamics. Furthermore, the report profiles leading market players and addresses critical drivers, restraints, opportunities, and challenges influencing the industry landscape, serving as a vital resource for strategic decision-making.
| Report Attributes | Report Details |
|---|---|
| Base Year | 2024 |
| Historical Year | 2019 to 2023 |
| Forecast Year | 2025 - 2033 |
| Market Size in 2025 | USD 95.5 Billion |
| Market Forecast in 2033 | USD 166.4 Billion |
| Growth Rate | 7.2% CAGR |
| Number of Pages | 265 |
| Key Trends |
|
| Segments Covered |
|
| Key Companies Covered | Roche Diagnostics, Abbott Laboratories, Siemens Healthineers, Danaher Corporation, Becton, Dickinson and Company, Sysmex Corporation, bioMérieux, Thermo Fisher Scientific, Qiagen, Illumina, Ortho Clinical Diagnostics, Hologic, Bio-Rad Laboratories, PerkinElmer, Grifols, Seegene, DiaSorin, Agilent Technologies, Quidel Corporation, Exact Sciences Corporation |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Speak to Analyst | Avail customised purchase options to meet your exact research needs. Request For Analyst Or Customization |
Segmentation Analysis
The In Vitro Diagnostic Device market is extensively segmented to provide a detailed understanding of its diverse components and their respective growth trajectories. This segmentation allows for precise analysis of market dynamics across various product types, technological innovations, clinical applications, and end-user adoption patterns. Such granular categorization is essential for identifying specific growth drivers, understanding competitive landscapes, and formulating targeted strategies within the highly specialized and evolving diagnostic industry. Each segment represents a unique set of market characteristics, influencing investment decisions and product development priorities.
- By Product Type: Includes Reagents & Kits, Instruments, and Software & Services, reflecting the comprehensive range of components required for IVD testing.
- By Technology: Covers major diagnostic methodologies such as Immunoassay, Clinical Chemistry, Molecular Diagnostics (further broken down into PCR, NGS, etc.), Hematology, Microbiology, Coagulation & Hemostasis, Urinalysis, and Other advanced techniques.
- By Application: Encompasses various therapeutic areas where IVD tests are crucial, including Infectious Diseases, Oncology, Cardiology, Diabetes, Nephrology, Autoimmune Diseases, Drug Testing, and other specialized applications.
- By End-User: Differentiates market consumption across key healthcare settings such as Hospitals & Clinics, Diagnostic Laboratories, Academic & Research Institutes, Home Care Settings, and other emerging diagnostic environments.
Regional Highlights
- North America: Dominates the market due to advanced healthcare infrastructure, high adoption of innovative technologies, significant research and development investments, and a favorable reimbursement landscape. The United States is a key contributor to this region's leadership.
- Europe: Represents a mature market with high awareness of early disease diagnosis, strong government initiatives for healthcare improvement, and a robust presence of key market players. Germany, the UK, and France are significant contributors.
- Asia Pacific (APAC): Expected to witness the highest growth rate, driven by a large patient pool, increasing healthcare expenditure, improving medical infrastructure, rising disposable incomes, and growing awareness regarding health and diagnostics in countries like China, India, and Japan.
- Latin America: Experiences steady growth owing to increasing investments in healthcare facilities, rising prevalence of chronic diseases, and a growing demand for accessible diagnostic solutions, particularly in Brazil and Mexico.
- Middle East and Africa (MEA): Emerging as a market with potential, supported by improving healthcare accessibility, government initiatives to modernize healthcare systems, and increasing foreign investments in the region, particularly in Saudi Arabia and UAE.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the In Vitro Diagnostic Device Market.- Roche Diagnostics
- Abbott Laboratories
- Siemens Healthineers
- Danaher Corporation
- Becton, Dickinson and Company
- Sysmex Corporation
- bioMérieux
- Thermo Fisher Scientific
- Qiagen
- Illumina
- Ortho Clinical Diagnostics
- Hologic
- Bio-Rad Laboratories
- PerkinElmer
- Grifols
- Seegene
- DiaSorin
- Agilent Technologies
- Quidel Corporation
- Exact Sciences Corporation
Frequently Asked Questions
What is the projected growth of the In Vitro Diagnostic Device market?
The In Vitro Diagnostic Device market is projected to grow at a Compound Annual Growth Rate (CAGR) of 7.2% from 2025 to 2033, reaching an estimated value of USD 166.4 Billion by 2033.
How is AI transforming the In Vitro Diagnostic Device industry?
AI is transforming the IVD industry by enhancing diagnostic accuracy through advanced data analysis, automating laboratory workflows, enabling predictive analytics for disease progression, and supporting the development of more personalized treatment strategies.
What are the primary drivers for the In Vitro Diagnostic Device market?
Key drivers include the increasing global prevalence of chronic and infectious diseases, the growing geriatric population, continuous technological advancements in diagnostic techniques, and a rising demand for personalized medicine approaches.
Which regions are leading the In Vitro Diagnostic Device market?
North America currently leads the In Vitro Diagnostic Device market due to its advanced healthcare infrastructure and technological adoption, while the Asia Pacific region is anticipated to exhibit the highest growth rate during the forecast period.
What are the main types of In Vitro Diagnostic Devices?
The main types of In Vitro Diagnostic Devices are segmented by product type to include Reagents and Kits, Instruments, and Software and Services, essential for various diagnostic tests.

