Hemophilia Treatment Market
Hemophilia Treatment Market Size, Scope, Growth, Trends and By Segmentation Types, Applications, Regional Analysis and Industry Forecast (2025-2033)
Report ID : RI_703849 | Last Updated : August 05, 2025 |
Format : ![]()
![]()
![]()
![]()
Hemophilia Treatment Market Size
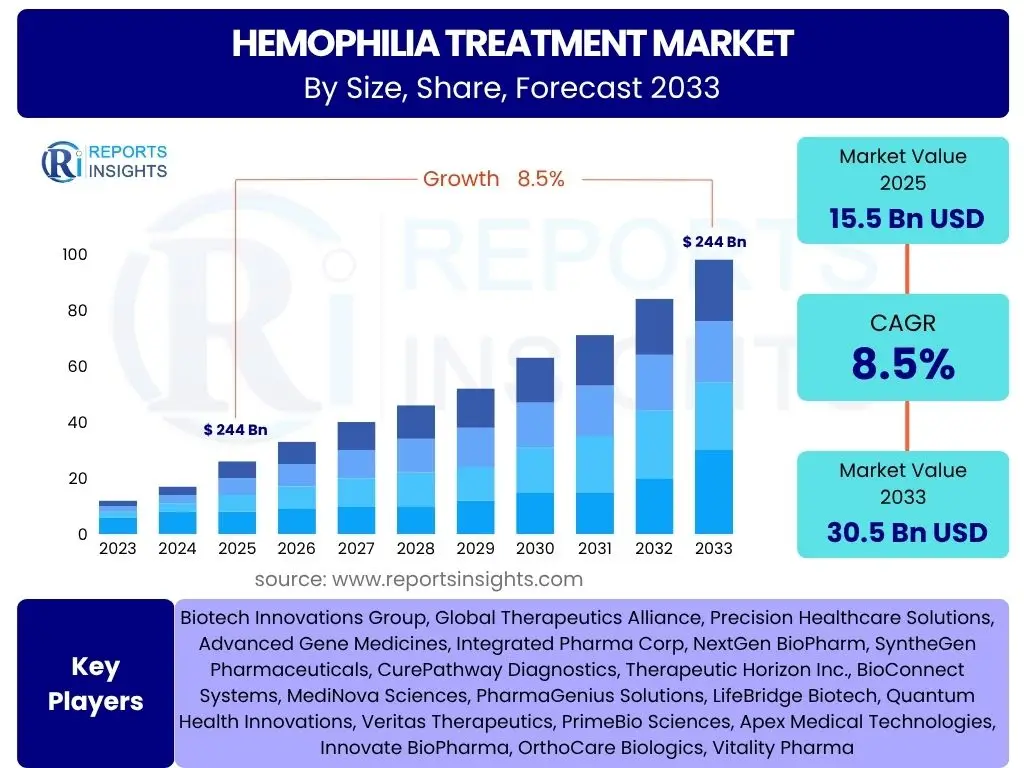
According to Reports Insights Consulting Pvt Ltd, The Hemophilia Treatment Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 8.5% between 2025 and 2033. The market is estimated at 15.5 Billion USD in 2025 and is projected to reach 30.5 Billion USD by the end of the forecast period in 2033.
Key Hemophilia Treatment Market Trends & Insights
The Hemophilia Treatment market is undergoing a significant transformation driven by the advent of novel therapeutic approaches, a shift towards prophylactic treatment, and an increasing global awareness of the condition. Advances in gene therapy are particularly notable, offering the potential for long-term or even curative solutions, moving beyond traditional factor replacement therapies. Furthermore, enhanced diagnostic capabilities and expanded screening programs are contributing to earlier detection and initiation of treatment, particularly in developing regions. The focus is also shifting towards personalized medicine, utilizing genetic profiling to tailor treatment regimens, thereby improving efficacy and reducing adverse events. This evolution ensures better patient outcomes and a higher quality of life for individuals with hemophilia.
- Emergence of novel non-factor replacement therapies, including gene therapies and rebalancing agents.
- Increased adoption of prophylactic treatment regimens to prevent bleeding episodes.
- Growing focus on personalized medicine and tailored treatment approaches based on patient profiles.
- Advancements in diagnostic technologies leading to earlier and more accurate diagnosis.
- Expansion of patient registries and real-world evidence studies to optimize treatment strategies.
AI Impact Analysis on Hemophilia Treatment
Artificial intelligence is poised to revolutionize hemophilia treatment by enhancing diagnostic accuracy, optimizing treatment protocols, and accelerating drug discovery. AI algorithms can analyze vast datasets of patient information, including genetic profiles, bleeding patterns, and treatment responses, to predict future bleeding events, inform personalized dosing, and identify patients at risk of developing inhibitors. This predictive capability allows for proactive intervention, potentially preventing severe complications and improving overall disease management. Furthermore, AI's application in drug development, from target identification to clinical trial design, promises to bring more effective and safer therapies to market faster, ultimately improving patient outcomes and reducing the overall burden of hemophilia.
- Predictive analytics for personalized bleeding risk assessment and prophylactic dosing.
- AI-driven drug discovery and development, accelerating the identification of novel therapeutic targets.
- Optimization of treatment regimens through analysis of patient-specific data.
- Enhanced diagnostic precision and early detection of hemophilia and its complications.
- Development of AI-powered remote monitoring and patient support systems for improved adherence and outcomes.
Key Takeaways Hemophilia Treatment Market Size & Forecast
The Hemophilia Treatment market is characterized by robust growth, primarily fueled by the continuous innovation in therapeutic options and increasing global efforts to improve access to care. A significant takeaway is the ongoing shift from on-demand treatment to prophylactic strategies, which is improving patient quality of life and reducing long-term complications. The market's future trajectory is heavily influenced by the successful commercialization of advanced therapies, particularly gene therapies, which represent a paradigm shift in managing the condition. Stakeholders should recognize the dual importance of investing in novel research while also focusing on expanding diagnostic capabilities and treatment access in underserved populations to sustain growth and address unmet medical needs effectively.
- Sustained market growth driven by therapeutic innovation and increasing prophylactic treatment adoption.
- Gene therapy and other non-factor therapies are set to redefine the treatment landscape.
- Emphasis on early diagnosis and improved access to comprehensive care, especially in emerging economies.
- Patient-centric approaches, including personalized medicine, are becoming central to treatment strategies.
- The high cost of advanced therapies remains a critical factor influencing market accessibility and growth.
Hemophilia Treatment Market Drivers Analysis
The primary drivers of the Hemophilia Treatment market include significant advancements in therapeutic options, particularly the development and increasing adoption of novel therapies beyond traditional factor replacement. This encompasses the progress in extended half-life (EHL) factors, non-factor replacement therapies, and transformative gene therapies, which promise more convenient dosing schedules and potentially curative outcomes. These innovations are enhancing the quality of life for patients and expanding the treatment landscape, driving higher market valuations. The demand for these advanced treatments is growing as healthcare providers and patients seek more effective and less burdensome solutions for managing this chronic condition.
Concurrently, the rising global prevalence and improved diagnosis rates of hemophilia are substantially contributing to market expansion. Enhanced awareness programs, improved diagnostic infrastructure, and increased screening in various regions, especially in emerging economies, are leading to a larger diagnosed patient pool requiring ongoing treatment. Government support and favorable reimbursement policies in developed countries also play a crucial role by making advanced and often expensive treatments accessible to a wider patient demographic. This supportive regulatory and reimbursement environment encourages pharmaceutical companies to invest further in research and development, fostering continuous innovation.
Furthermore, the shift towards prophylactic treatment regimens, particularly in pediatric patients, is a significant growth driver. Prophylaxis, aimed at preventing bleeding episodes, is recognized as the gold standard of care, leading to reduced joint damage and improved long-term outcomes. The increasing adherence to these regimens translates into consistent demand for factor concentrates and novel therapies. This paradigm shift, coupled with an aging hemophilia population due to better treatment, ensures a sustained demand for effective management solutions, thereby reinforcing market growth.
| Drivers | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Advancements in Gene Therapy and Novel Therapeutics | +1.2% | Global, particularly North America, Europe | Long-term (2025-2033) |
| Rising Global Prevalence and Improved Diagnosis Rates | +0.9% | Asia Pacific, Latin America, Europe | Mid to Long-term (2025-2030) |
| Increasing Adoption of Prophylactic Treatment Regimens | +0.7% | North America, Europe, select APAC countries | Short to Mid-term (2025-2028) |
| Favorable Government Support and Reimbursement Policies | +0.6% | North America, Western Europe | Long-term (2025-2033) |
Hemophilia Treatment Market Restraints Analysis
The high cost associated with hemophilia treatment, particularly for novel and advanced therapies like gene therapy, represents a significant restraint on market growth. These therapies often involve substantial upfront costs, making them inaccessible for a considerable portion of the global patient population, especially in low and middle-income countries with limited healthcare budgets and less robust insurance coverage. The economic burden on healthcare systems and individual patients can limit the widespread adoption of the most effective treatments, hindering market expansion. This cost factor necessitates complex negotiations with payers and can lead to disparities in treatment access globally.
Limited access to diagnostic facilities and treatment in developing regions also acts as a major impediment. Many parts of the world lack the necessary infrastructure, trained medical professionals, and consistent supply chains to diagnose hemophilia accurately and deliver continuous, high-quality care. This diagnostic gap means a large number of patients remain undiagnosed or undertreated, preventing them from accessing available therapies and consequently limiting the overall market size. Efforts to expand access are ongoing, but progress is slow in many critical regions, impacting the global penetration of advanced therapies.
The development of inhibitors to factor replacement therapies is another clinical restraint. A significant percentage of patients, particularly those with severe hemophilia A, develop antibodies (inhibitors) against infused factor VIII, rendering standard replacement therapy ineffective. Managing these patients requires expensive bypass agents or immune tolerance induction (ITI) therapy, which adds to the treatment complexity and cost. The challenge of inhibitor development not only affects treatment efficacy but also imposes a substantial economic burden, influencing treatment choices and patient outcomes, thus impacting market dynamics and limiting the broad applicability of certain treatment modalities.
| Restraints | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| High Cost of Advanced Therapies and Treatment | -0.8% | Global, particularly emerging markets | Long-term (2025-2033) |
| Limited Access to Diagnosis and Treatment in Developing Regions | -0.6% | Asia Pacific, Latin America, MEA | Long-term (2025-2033) |
| Development of Inhibitors to Factor Replacement Therapies | -0.4% | Global | Mid to Long-term (2025-2030) |
| Stringent Regulatory Approval Processes for Novel Therapies | -0.3% | North America, Europe | Short to Mid-term (2025-2028) |
Hemophilia Treatment Market Opportunities Analysis
The significant unmet medical need in emerging economies presents a substantial growth opportunity for the Hemophilia Treatment market. These regions often have large undiagnosed or undertreated patient populations due to a lack of awareness, insufficient diagnostic capabilities, and limited healthcare infrastructure. As economic development progresses and healthcare expenditure increases in these countries, there will be a growing demand for effective hemophilia treatments. Companies focusing on expanding their presence and improving access in these markets, potentially through tiered pricing or local manufacturing, can unlock considerable untapped potential and drive significant market expansion.
Advancements in personalized medicine and gene editing technologies offer transformative opportunities for future market growth. Tailoring treatment based on an individual patient's genetic profile and disease characteristics can lead to more effective and safer therapies, maximizing clinical benefit and reducing adverse effects. Gene editing, while still in early stages for clinical application in hemophilia, holds the promise of precise genetic corrections, potentially leading to permanent cures. Investments in these cutting-edge fields will drive the next wave of innovation and patient stratification, opening new revenue streams and expanding therapeutic possibilities, thereby shaping the future of hemophilia care.
The increasing focus on home-based care and digital health solutions represents another compelling opportunity. For a chronic condition like hemophilia, allowing patients to administer treatment at home improves convenience, reduces hospital visits, and enhances adherence to treatment regimens. Digital platforms for remote monitoring, teleconsultation, and treatment adherence tracking can further optimize care delivery. These solutions not only improve patient quality of life but also offer cost efficiencies for healthcare systems, making them attractive avenues for market expansion and value creation. The post-pandemic environment has accelerated the adoption of such models, solidifying their potential to revolutionize how hemophilia care is delivered globally.
| Opportunities | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Untapped Market Potential in Emerging Economies | +1.0% | Asia Pacific, Latin America, MEA | Long-term (2025-2033) |
| Advancements in Personalized Medicine and Gene Editing Technologies | +0.9% | Global, particularly developed nations | Long-term (2028-2033) |
| Growing Adoption of Home-Based Care and Digital Health Solutions | +0.7% | North America, Europe, high-income APAC | Mid to Long-term (2025-2030) |
| Development of Novel Therapies for Inhibitor Patients | +0.6% | Global | Mid-term (2025-2028) |
Hemophilia Treatment Market Challenges Impact Analysis
Navigating the complex regulatory landscape and securing market approval for novel hemophilia treatments, particularly gene therapies, poses a significant challenge. Regulatory bodies demand extensive clinical trial data demonstrating both efficacy and long-term safety, given the irreversible nature of some advanced therapies. The process is time-consuming and resource-intensive, often leading to delays in market entry. Different regulatory requirements across countries add to the complexity, necessitating tailored strategies for global commercialization, which can slow down patient access to innovative treatments and impact market growth projections.
Ethical considerations and public acceptance surrounding gene therapy represent a notable challenge. Concerns exist regarding the long-term safety, potential off-target effects, and the moral implications of altering the human genome, even for therapeutic purposes. Ensuring informed consent, managing patient expectations, and addressing societal anxieties are critical for the successful adoption and integration of these transformative treatments into mainstream medical practice. Public skepticism or resistance, if not adequately addressed through transparent communication and robust safety data, could impede market penetration and limit the overall impact of these promising therapies.
Ensuring robust cold chain logistics for biological products, which constitute a significant portion of hemophilia treatments, presents a perpetual operational challenge. Factor concentrates and many novel therapies are temperature-sensitive, requiring precise storage and transportation conditions to maintain their efficacy and safety. Any deviation from these conditions can render the product ineffective or even harmful. This logistical complexity adds to the cost of distribution and limits accessibility in regions with underdeveloped infrastructure or extreme climates, particularly impacting global supply chain efficiency and the ability to reach all patients in need of these vital treatments.
| Challenges | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Stringent Regulatory Approval and Market Access for Advanced Therapies | -0.5% | North America, Europe | Long-term (2025-2033) |
| Ethical Concerns and Public Acceptance of Gene Therapy | -0.3% | Global | Long-term (2025-2033) |
| Maintaining Cold Chain Logistics for Biological Products | -0.2% | Developing Regions, Remote Areas | Ongoing (2025-2033) |
| Reimbursement Challenges and Payer Negotiations for High-Cost Therapies | -0.4% | Global | Long-term (2025-2033) |
Hemophilia Treatment Market - Updated Report Scope
This comprehensive report on the Hemophilia Treatment Market provides an in-depth analysis of market dynamics, segmentation, regional insights, and the competitive landscape. It offers a detailed forecast from 2025 to 2033, evaluating key trends, drivers, restraints, opportunities, and challenges influencing market growth. The study encompasses a thorough review of historical market performance and projections for future expansion, incorporating the impact of technological advancements, particularly in gene therapy and AI, on treatment paradigms. The scope also includes an assessment of various product types, routes of administration, and end-user segments, alongside a profile of major market players to offer a holistic view of the industry, enabling strategic decision-making for stakeholders.
| Report Attributes | Report Details |
|---|---|
| Base Year | 2024 |
| Historical Year | 2019 to 2023 |
| Forecast Year | 2025 - 2033 |
| Market Size in 2025 | 15.5 Billion USD |
| Market Forecast in 2033 | 30.5 Billion USD |
| Growth Rate | 8.5% |
| Number of Pages | 255 |
| Key Trends |
|
| Segments Covered |
|
| Key Companies Covered | Biotech Innovations Group, Global Therapeutics Alliance, Precision Healthcare Solutions, Advanced Gene Medicines, Integrated Pharma Corp, NextGen BioPharm, SyntheGen Pharmaceuticals, CurePathway Diagnostics, Therapeutic Horizon Inc., BioConnect Systems, MediNova Sciences, PharmaGenius Solutions, LifeBridge Biotech, Quantum Health Innovations, Veritas Therapeutics, PrimeBio Sciences, Apex Medical Technologies, Innovate BioPharma, OrthoCare Biologics, Vitality Pharma |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Speak to Analyst | Avail customised purchase options to meet your exact research needs. Request For Analyst Or Customization |
Segmentation Analysis
The Hemophilia Treatment Market is segmented across various dimensions to provide a granular understanding of its components and growth drivers. These segments include classifications based on the type of hemophilia (A, B, or other rarer forms), the specific product types utilized for treatment (factor concentrates, bypass agents, and novel therapies), the route of administration (intravenous or subcutaneous), the primary distribution channels (hospital, retail, online pharmacies, and specialized treatment centers), and the end-user facilities (hospitals, clinics, and homecare settings). This detailed segmentation allows for a comprehensive analysis of patient needs, therapeutic preferences, and market penetration across different categories, highlighting areas of high growth potential and unmet clinical demand. Understanding these segments is crucial for strategic planning and resource allocation within the market to effectively address diverse patient populations and healthcare systems globally.
- By Type of Hemophilia: Hemophilia A, Hemophilia B, Other Hemophilias.
- By Product Type: Factor VIII Concentrates, Factor IX Concentrates, Bypass Agents, Novel Therapies.
- By Route of Administration: Intravenous, Subcutaneous.
- By Distribution Channel: Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Hemophilia Treatment Centers.
- By End-User: Hospitals, Clinics, Homecare Settings.
Regional Highlights
- North America: Dominates the market due to high adoption of advanced therapies, robust healthcare infrastructure, and favorable reimbursement policies. Significant research and development investment and a large diagnosed patient population contribute to its leading position in treatment innovation and market value.
- Europe: A key region driven by strong government support for rare disease treatments, high awareness levels, and continuous advancements in gene therapy research. Countries such as Germany, France, and the UK are prominent contributors to market growth, benefiting from established hemophilia care networks and patient advocacy.
- Asia Pacific (APAC): Expected to witness the fastest growth due to increasing healthcare expenditure, improving diagnostic capabilities, and a large untapped patient pool. Rising awareness, government initiatives to improve access to care, and the burgeoning medical tourism sector are driving expansion in countries like China, India, and Japan.
- Latin America: Demonstrates steady growth, propelled by increasing awareness, improving economic conditions, and concerted efforts to enhance access to hemophilia care. Brazil and Mexico are key markets in this region, driven by growing healthcare investments and patient support programs.
- Middle East and Africa (MEA): Represents an emerging market with significant growth potential, driven by developing healthcare infrastructure, increasing investments in healthcare, and a rising prevalence of inherited disorders. However, challenges related to affordability and widespread access to specialized care remain important factors influencing market penetration.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Hemophilia Treatment Market.- Biotech Innovations Group
- Global Therapeutics Alliance
- Precision Healthcare Solutions
- Advanced Gene Medicines
- Integrated Pharma Corp
- NextGen BioPharm
- SyntheGen Pharmaceuticals
- CurePathway Diagnostics
- Therapeutic Horizon Inc.
- BioConnect Systems
- MediNova Sciences
- PharmaGenius Solutions
- LifeBridge Biotech
- Quantum Health Innovations
- Veritas Therapeutics
- PrimeBio Sciences
- Apex Medical Technologies
- Innovate BioPharma
- OrthoCare Biologics
- Vitality Pharma
Frequently Asked Questions
What are the latest advancements in Hemophilia Treatment?
Recent advancements include the development of extended half-life factor concentrates, innovative non-factor replacement therapies such as bi-specific antibodies and RNA interference agents, and transformative gene therapies offering the potential for long-term correction of factor deficiencies. These innovations aim to reduce treatment burden and improve patient quality of life.
How is Artificial Intelligence (AI) impacting the Hemophilia Treatment market?
AI is impacting hemophilia treatment by enabling personalized dosing, predicting bleeding episodes, accelerating drug discovery for novel therapies, and optimizing patient management through data analysis. It enhances diagnostic precision and supports remote monitoring, leading to more efficient and tailored care.
What are the primary drivers of growth in the Hemophilia Treatment market?
Key growth drivers include the continuous innovation in gene therapy and novel non-factor replacement therapies, increasing global diagnosis rates, and the growing adoption of prophylactic treatment regimens. Favorable government support and reimbursement policies in developed nations also significantly contribute to market expansion.
What challenges are faced by the Hemophilia Treatment market?
Major challenges include the high cost of advanced therapies, which limits accessibility in many regions, complex regulatory approval processes for novel treatments, and the logistical challenges associated with maintaining cold chain for biological products. Ethical concerns surrounding gene therapy also present a challenge to widespread adoption.
What are the key opportunities for stakeholders in the Hemophilia Treatment market?
Significant opportunities lie in untapped markets within emerging economies, further advancements in personalized medicine and gene editing technologies, and the expansion of home-based care and digital health solutions. Developing novel therapies specifically for inhibitor patients also represents a crucial area for growth and innovation.