Biopharmaceutical Market
Biopharmaceutical Market Size, Scope, Growth, Trends and By Segmentation Types, Applications, Regional Analysis and Industry Forecast (2025-2033)
Report ID : RI_702162 | Last Updated : July 31, 2025 |
Format : ![]()
![]()
![]()
![]()
Biopharmaceutical Market Size
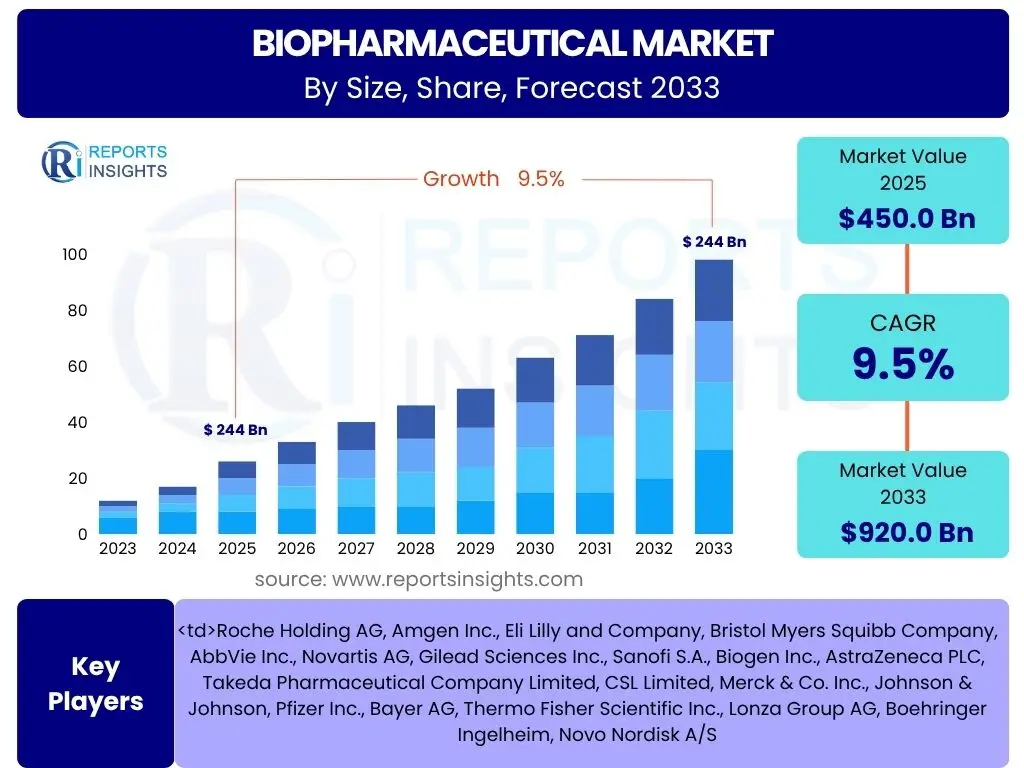
According to Reports Insights Consulting Pvt Ltd, The Biopharmaceutical Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 9.5% between 2025 and 2033. The market is estimated at USD 450.0 Billion in 2025 and is projected to reach USD 920.0 Billion by the end of the forecast period in 2033.
Key Biopharmaceutical Market Trends & Insights
The biopharmaceutical market is undergoing a significant transformation, driven by an accelerating pace of scientific discovery and evolving healthcare needs. Common user inquiries often revolve around the primary forces shaping this evolution, including the shift towards advanced therapeutic modalities, the impact of digitalization, and changes in drug development paradigms. Key insights reveal a robust pipeline of novel biologics, the increasing prevalence of chronic and rare diseases, and a strategic focus on personalized medicine approaches, all contributing to sustained market expansion.
Another crucial trend captivating industry attention is the globalization of research and development, alongside the rise of contract manufacturing and research organizations. Stakeholders are keen to understand how these trends influence market access, cost efficiencies, and overall drug commercialization. The convergence of biotechnology with digital health solutions is also reshaping patient engagement and treatment delivery, promising more efficient and targeted therapeutic interventions.
- Accelerated Shift Towards Biologics and Biosimilars: Continued dominance and growth in the biologics segment, coupled with the increasing availability and adoption of cost-effective biosimilars.
- Advancements in Gene and Cell Therapies: Rapid development and regulatory approvals for innovative therapies targeting previously untreatable diseases.
- Integration of Artificial Intelligence and Machine Learning: Growing application of AI/ML in drug discovery, clinical trial design, and personalized medicine.
- Emphasis on Personalized and Precision Medicine: Tailoring treatments to individual patient genetic profiles for improved efficacy and reduced adverse effects.
- Growth in Orphan Drugs and Rare Disease Treatments: Increased focus on developing therapies for underserved rare disease populations, often benefiting from expedited regulatory pathways.
- Strategic Collaborations and Outsourcing: Pharmaceutical companies increasingly partnering with Contract Development and Manufacturing Organizations (CDMOs) and Contract Research Organizations (CROs) for R&D and manufacturing efficiencies.
- Digital Transformation in Clinical Trials: Adoption of decentralized clinical trials and digital tools to enhance patient recruitment, data collection, and overall trial efficiency.
AI Impact Analysis on Biopharmaceutical
Common user questions regarding AI's influence on the biopharmaceutical sector frequently address its potential to revolutionize drug discovery, optimize clinical trials, and enhance patient outcomes. Users are keen to understand how AI tools are being deployed to accelerate the identification of drug targets, predict molecular interactions, and streamline preclinical research, ultimately reducing the time and cost associated with bringing new therapies to market. There is significant interest in AI's role in processing vast datasets, including genomic and proteomic information, to uncover new insights into disease mechanisms.
Furthermore, concerns and expectations often center on AI's capacity to improve the efficiency and success rates of clinical development. This includes AI-driven patient selection, real-time monitoring of trial participants, and predictive analytics for trial outcomes. While the promise of AI for personalized medicine and precision dosing is widely acknowledged, questions also arise about data privacy, algorithm bias, and the necessity for robust validation frameworks to ensure reliable and ethical deployment of AI technologies within a highly regulated industry. The industry anticipates AI to be a core pillar of future drug development strategies.
- Accelerated Drug Discovery and Target Identification: AI algorithms analyze vast datasets to identify novel drug candidates and therapeutic targets, significantly reducing early-stage research time.
- Enhanced Clinical Trial Efficiency: AI optimizes patient recruitment, predicts trial outcomes, and monitors patient responses in real-time, leading to faster and more cost-effective clinical development.
- Improved Biomarker Discovery: AI identifies specific biological indicators for disease progression or treatment response, crucial for personalized medicine and diagnostic development.
- Optimized Manufacturing and Quality Control: AI enables predictive maintenance of equipment, optimizes fermentation processes, and enhances quality assurance in biopharmaceutical production.
- Personalized Medicine and Patient Stratification: AI analyzes individual patient data to predict treatment efficacy and adverse reactions, facilitating tailored therapeutic approaches.
- Data Integration and Analysis: AI tools process and integrate diverse biological, clinical, and real-world data, unlocking deeper insights and informing strategic decisions.
Key Takeaways Biopharmaceutical Market Size & Forecast
Analyzing common user questions about the biopharmaceutical market's size and forecast reveals a keen interest in understanding the underlying drivers of its projected growth and the segments poised for significant expansion. Stakeholders consistently inquire about the sustainability of the market's high growth trajectory, the influence of emerging therapeutic areas, and the potential impact of global healthcare spending trends. The primary insight is a robust and sustained growth outlook, largely fueled by a burgeoning pipeline of innovative biologic drugs and the increasing prevalence of chronic and complex diseases worldwide.
Furthermore, inquiries often highlight the importance of geographical contributions, with particular attention paid to the evolving roles of established markets versus rapidly expanding emerging economies. The market forecast underscores the continued dominance of novel therapeutic modalities, such as gene and cell therapies, as significant contributors to market value. These trends collectively suggest that strategic investments in R&D, market access, and advanced manufacturing capabilities will be critical for companies aiming to capitalize on the sustained expansion of the biopharmaceutical sector through 2033.
- Strong Growth Momentum: The market is projected to nearly double its value from 2025 to 2033, indicating robust and sustained expansion.
- Innovation-Driven Expansion: Growth is primarily fueled by the continuous development and commercialization of advanced biologics, gene therapies, and cell therapies.
- Disease Burden as a Catalyst: Increasing global prevalence of chronic diseases, autoimmune disorders, and cancer drives the demand for biopharmaceutical treatments.
- Strategic Investments in R&D: Significant investments in research and development by pharmaceutical companies and biotechnological firms underpin pipeline strength.
- Global Market Reach: While North America and Europe remain dominant, emerging markets in Asia Pacific and Latin America are poised for accelerated growth, contributing significantly to overall market size.
Biopharmaceutical Market Drivers Analysis
The biopharmaceutical market's expansion is fundamentally propelled by a confluence of demographic shifts, scientific breakthroughs, and supportive regulatory environments. The global aging population, coupled with a rising incidence of chronic and complex diseases such as cancer, autoimmune disorders, and diabetes, creates an ever-increasing demand for advanced therapeutic solutions. This demographic imperative drives both the discovery of new drugs and the broader adoption of existing ones, ensuring a sustained need for innovative biopharmaceutical products. Furthermore, the inherent complexity and efficacy of biologics often position them as preferred treatment options where conventional small-molecule drugs fall short.
Technological advancements are another critical catalyst, pushing the boundaries of what is medically possible. Innovations in genetic engineering, protein expression systems, and cell culture technologies enable the development of highly specific and potent biopharmaceuticals, including monoclonal antibodies, gene therapies, and cell therapies. These scientific leaps are complemented by substantial and growing investments in research and development across both public and private sectors. Such funding accelerates preclinical and clinical studies, expanding the pipeline of potential future therapies and ensuring a steady flow of novel products to address unmet medical needs. Additionally, evolving regulatory frameworks, often providing expedited pathways for breakthrough therapies, further incentivize investment and innovation in the biopharmaceutical space.
| Drivers | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Aging Population and Rising Chronic Disease Prevalence | +2.5% | Global | Short to Mid-term (2025-2030) |
| Technological Advancements in Biologics and Gene Therapies | +2.0% | North America, Europe, Asia Pacific | Long-term (2025-2033) |
| Increased Research and Development (R&D) Investments | +1.5% | Global | Mid to Long-term (2026-2033) |
| Favorable Regulatory Pathways for Novel Therapies | +1.0% | North America, Europe | Mid-term (2025-2030) |
Biopharmaceutical Market Restraints Analysis
Despite its robust growth, the biopharmaceutical market faces several significant restraints that could temper its expansion. One of the most prominent challenges is the exceptionally high cost associated with the development, manufacturing, and commercialization of biopharmaceutical products. The complex R&D processes, coupled with rigorous clinical trials and specialized manufacturing facilities, require substantial capital investment, which translates into high product prices. These elevated costs can limit patient access and put considerable pressure on healthcare systems globally, leading to increased scrutiny from payers and policymakers regarding reimbursement and pricing strategies.
Furthermore, the stringent and often protracted regulatory approval processes across different regions pose a considerable hurdle. Biopharmaceuticals, due to their intricate nature and potential immunogenicity, undergo exhaustive safety and efficacy evaluations, which can extend the time-to-market and increase development risks. Another significant restraint is the impending patent expirations of several blockbuster biologics, which will inevitably lead to increased competition from biosimilars. While biosimilars offer cost-effective alternatives, their market entry can erode the revenue streams of originator products, compelling companies to continuously innovate to maintain market share. Supply chain vulnerabilities, exacerbated by global events, also represent a persistent challenge, impacting the timely delivery of critical raw materials and finished products, thereby affecting production and distribution timelines.
| Restraints | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| High Cost of Research, Development, and Manufacturing | -1.5% | Global | Ongoing |
| Stringent and Lengthy Regulatory Approval Processes | -1.0% | Global | Ongoing |
| Patent Expirations and Biosimilar Competition | -0.8% | North America, Europe | Mid to Long-term (2027-2033) |
| Supply Chain Vulnerabilities and Geopolitical Risks | -0.5% | Global | Short-term (2025-2027) |
Biopharmaceutical Market Opportunities Analysis
The biopharmaceutical market is ripe with opportunities that promise to drive substantial future growth and reshape the healthcare landscape. A significant avenue for expansion lies in the exploration of emerging markets, particularly in Asia Pacific, Latin America, and parts of Africa. These regions represent vast, untapped patient populations with rising healthcare expenditures and improving access to advanced medical treatments. Companies that strategically invest in establishing a strong presence and adapting to local regulatory and economic nuances in these areas can unlock significant new revenue streams and diversify their market base.
Another transformative opportunity is the continued advancement and broader adoption of personalized medicine, including gene and cell therapies. These highly specialized treatments offer unprecedented potential for curing diseases at their genetic roots or providing highly effective therapies for previously intractable conditions. The increasing understanding of individual patient biology, coupled with sophisticated diagnostic tools, enables the development of bespoke therapies, thereby expanding the addressable market for targeted biopharmaceuticals. Furthermore, strategic collaborations and partnerships, ranging from academic research institutions to contract manufacturing organizations, offer opportunities for shared risk, accelerated development, and expanded capabilities. The ongoing digital transformation within healthcare, leveraging data analytics, artificial intelligence, and real-world evidence, also presents significant opportunities to optimize drug development, improve patient engagement, and enhance commercialization strategies.
| Opportunities | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Expansion into Emerging Markets (e.g., Asia Pacific, Latin America) | +1.8% | Asia Pacific, Latin America | Long-term (2028-2033) |
| Further Development and Commercialization of Personalized Medicine, Gene, and Cell Therapies | +2.2% | North America, Europe | Long-term (2027-2033) |
| Strategic Collaborations, Acquisitions, and Partnerships | +1.5% | Global | Mid to Long-term (2026-2033) |
| Leveraging Digital Transformation and Data Analytics for R&D and Commercialization | +1.2% | Global | Mid to Long-term (2026-2033) |
Biopharmaceutical Market Challenges Impact Analysis
The biopharmaceutical market, while innovative, faces a complex array of challenges that can impede its growth and operational efficiency. One of the most persistent issues is the escalating pressure on drug pricing and the evolving landscape of reimbursement policies. Healthcare systems and payers globally are increasingly scrutinizing the high costs of biopharmaceuticals, leading to stricter formulary inclusions, value-based pricing models, and direct negotiation efforts. This pressure not only affects profitability but also complicates market access for novel therapies, particularly in cost-sensitive regions. Companies must continually demonstrate the long-term clinical and economic value of their products to secure favorable reimbursement.
Another significant challenge stems from the inherent complexity of biopharmaceutical manufacturing processes. Unlike small-molecule drugs, biologics require living systems for production, demanding highly specialized facilities, rigorous quality control, and sophisticated purification techniques. This complexity contributes to higher production costs, potential scalability issues, and a greater susceptibility to manufacturing deviations, which can lead to supply disruptions. Furthermore, the biopharmaceutical sector grapples with a talent shortage, particularly in highly specialized areas such as gene therapy development, bioinformatics, and advanced manufacturing. The scarcity of skilled professionals can hinder research progress, slow down clinical trials, and limit production capacities. Finally, as the industry increasingly relies on digital technologies and data, concerns around data security and patient privacy have escalated. Protecting sensitive health information and intellectual property from cyber threats and ensuring compliance with stringent data protection regulations (like GDPR and HIPAA) remains a critical and ongoing challenge that requires continuous investment and vigilance.
| Challenges | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Increasing Pricing Pressures and Reimbursement Challenges | -1.2% | North America, Europe | Ongoing |
| Complexity and High Costs of Biopharmaceutical Manufacturing | -0.7% | Global | Ongoing |
| Talent Shortages in Specialized Biopharmaceutical Fields | -0.6% | Global | Mid-term (2025-2030) |
| Data Security, Privacy, and Regulatory Compliance Concerns | -0.4% | Global | Ongoing |
Biopharmaceutical Market - Updated Report Scope
This comprehensive report provides an in-depth analysis of the global biopharmaceutical market, offering detailed insights into market dynamics, segmentation, regional landscapes, and competitive strategies. The scope encompasses a thorough examination of market size and forecast, drivers, restraints, opportunities, and challenges influencing industry growth from 2025 to 2033. It also delves into the impact of key technological advancements, such as artificial intelligence, and profiles leading market players to provide a holistic view of the market ecosystem.
| Report Attributes | Report Details |
|---|---|
| Base Year | 2024 |
| Historical Year | 2019 to 2023 |
| Forecast Year | 2025 - 2033 |
| Market Size in 2025 | USD 450.0 Billion |
| Market Forecast in 2033 | USD 920.0 Billion |
| Growth Rate | 9.5% |
| Number of Pages | 257 |
| Key Trends |
|
| Segments Covered |
|
| Key Companies Covered | Roche Holding AG, Amgen Inc., Eli Lilly and Company, Bristol Myers Squibb Company, AbbVie Inc., Novartis AG, Gilead Sciences Inc., Sanofi S.A., Biogen Inc., AstraZeneca PLC, Takeda Pharmaceutical Company Limited, CSL Limited, Merck & Co. Inc., Johnson & Johnson, Pfizer Inc., Bayer AG, Thermo Fisher Scientific Inc., Lonza Group AG, Boehringer Ingelheim, Novo Nordisk A/S |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Speak to Analyst | Avail customised purchase options to meet your exact research needs. Request For Analyst Or Customization |
Segmentation Analysis
The global biopharmaceutical market is intricately segmented across various dimensions, reflecting the diversity of its products, applications, manufacturing processes, and distribution channels. This granular segmentation provides a comprehensive understanding of the market's structure and allows for targeted analysis of specific growth pockets. The classification by product type highlights the dominance of complex biologics, while application-based segmentation reveals the critical therapeutic areas driving demand, such as oncology and autoimmune diseases, which continue to represent significant unmet medical needs.
Further segmentations by manufacturing type and distribution channel shed light on the operational landscape and market access dynamics. The growing trend of outsourcing to Contract Development and Manufacturing Organizations (CDMOs) signifies a strategic shift towards specialized expertise and cost efficiency, while the varied distribution pathways underscore the complex journey of biopharmaceuticals from production to patient. Each segment offers unique growth trajectories and competitive dynamics, providing valuable insights for market participants and strategic planning.
- By Product Type:
- Monoclonal Antibodies (mAbs)
- Recombinant Proteins (e.g., Insulin, Growth Hormones, Interferons)
- Vaccines (e.g., Therapeutic, Prophylactic)
- Gene & Cell Therapies (e.g., CAR-T, AAV-based therapies)
- Oligonucleotides
- Blood Plasma Derivatives
- Others (e.g., Peptides, Enzymes)
- By Application:
- Oncology
- Autoimmune Diseases
- Infectious Diseases
- Cardiovascular Diseases
- Neurological Disorders
- Metabolic Disorders (e.g., Diabetes)
- Rare Diseases
- Others (e.g., Ophthalmology, Dermatology)
- By Manufacturing Type:
- In-house Manufacturing
- Outsourced Manufacturing (CMOs/CDMOs)
- By Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Regional Highlights
The global biopharmaceutical market exhibits significant regional disparities in terms of market size, growth trajectory, and strategic importance. North America and Europe currently represent the largest and most mature markets, driven by robust R&D infrastructure, advanced healthcare systems, high prevalence of chronic diseases, and substantial healthcare expenditure. These regions also benefit from favorable regulatory frameworks and a strong presence of key market players, leading to continuous innovation and rapid adoption of novel therapies.
Asia Pacific is emerging as the fastest-growing region, fueled by expanding patient populations, increasing healthcare investments, improving economic conditions, and rising awareness about advanced treatments. Countries like China, India, and Japan are becoming attractive destinations for clinical trials and manufacturing, while also witnessing a surge in domestic biopharmaceutical production and consumption. Latin America, the Middle East, and Africa are also poised for growth, albeit at a slower pace, as healthcare infrastructure improves and access to specialized medicines expands in these developing economies. Each region presents unique opportunities and challenges, requiring tailored market entry and growth strategies.
- North America: Dominates the global biopharmaceutical market due to high R&D investments, advanced healthcare infrastructure, high disease prevalence, and a strong presence of leading pharmaceutical and biotechnology companies. The United States is a key contributor to market growth and innovation.
- Europe: A significant market driven by an aging population, rising chronic disease burden, robust regulatory support, and strong research capabilities. Countries such as Germany, France, and the UK are major contributors to biopharmaceutical innovation and consumption.
- Asia Pacific (APAC): Expected to be the fastest-growing region, propelled by large and aging populations, increasing healthcare expenditure, improving access to healthcare, and a growing focus on pharmaceutical manufacturing and R&D in countries like China, India, and Japan.
- Latin America: Demonstrating steady growth due to increasing awareness of advanced therapies, improving healthcare access, and rising prevalence of chronic diseases. Brazil and Mexico are key markets in this region.
- Middle East and Africa (MEA): Emerging markets with potential growth driven by rising healthcare investments, improving infrastructure, and efforts to diversify economies beyond oil. However, growth might be constrained by socio-economic disparities and political instability in certain areas.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Biopharmaceutical Market.- Roche Holding AG
- Amgen Inc.
- Eli Lilly and Company
- Bristol Myers Squibb Company
- AbbVie Inc.
- Novartis AG
- Gilead Sciences Inc.
- Sanofi S.A.
- Biogen Inc.
- AstraZeneca PLC
- Takeda Pharmaceutical Company Limited
- CSL Limited
- Merck & Co. Inc.
- Johnson & Johnson
- Pfizer Inc.
- Bayer AG
- Thermo Fisher Scientific Inc.
- Lonza Group AG
- Boehringer Ingelheim
- Novo Nordisk A/S
Frequently Asked Questions
Analyze common user questions about the Biopharmaceutical market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is the projected growth of the biopharmaceutical market?
The biopharmaceutical market is projected to grow at a Compound Annual Growth Rate (CAGR) of 9.5% between 2025 and 2033, reaching an estimated USD 920.0 Billion by 2033 from USD 450.0 Billion in 2025. This robust growth is driven by innovation in biologics and increasing global demand for advanced therapies.
How is AI transforming the biopharmaceutical industry?
Artificial intelligence (AI) is significantly transforming the biopharmaceutical industry by accelerating drug discovery, optimizing clinical trial design and execution, and enhancing personalized medicine approaches. AI tools improve target identification, streamline data analysis, and enable more efficient manufacturing processes, reducing development timelines and costs.
What are the primary drivers of the biopharmaceutical market?
Key drivers include the global aging population, rising prevalence of chronic and complex diseases, continuous technological advancements in biologics and gene therapies, and increasing investments in research and development. Favorable regulatory pathways for breakthrough therapies also play a crucial role in market expansion.
What challenges does the biopharmaceutical market face?
The biopharmaceutical market faces challenges such as the high cost of R&D and manufacturing, stringent regulatory approval processes, increasing pricing pressures and reimbursement challenges, and potential impacts from patent expirations and biosimilar competition. Supply chain vulnerabilities and talent shortages also present ongoing hurdles.
Which regions are key players in the global biopharmaceutical market?
North America and Europe are the largest and most mature markets due to advanced healthcare systems and significant R&D investments. Asia Pacific is the fastest-growing region, driven by large populations and increasing healthcare expenditure. Latin America and MEA are emerging markets with growing potential.