Biopharmaceutical Processing Seal Market
Biopharmaceutical Processing Seal Market Size, Scope, Growth, Trends and By Segmentation Types, Applications, Regional Analysis and Industry Forecast (2025-2033)
Report ID : RI_702294 | Last Updated : July 31, 2025 |
Format : ![]()
![]()
![]()
![]()
Biopharmaceutical Processing Seal Market Size
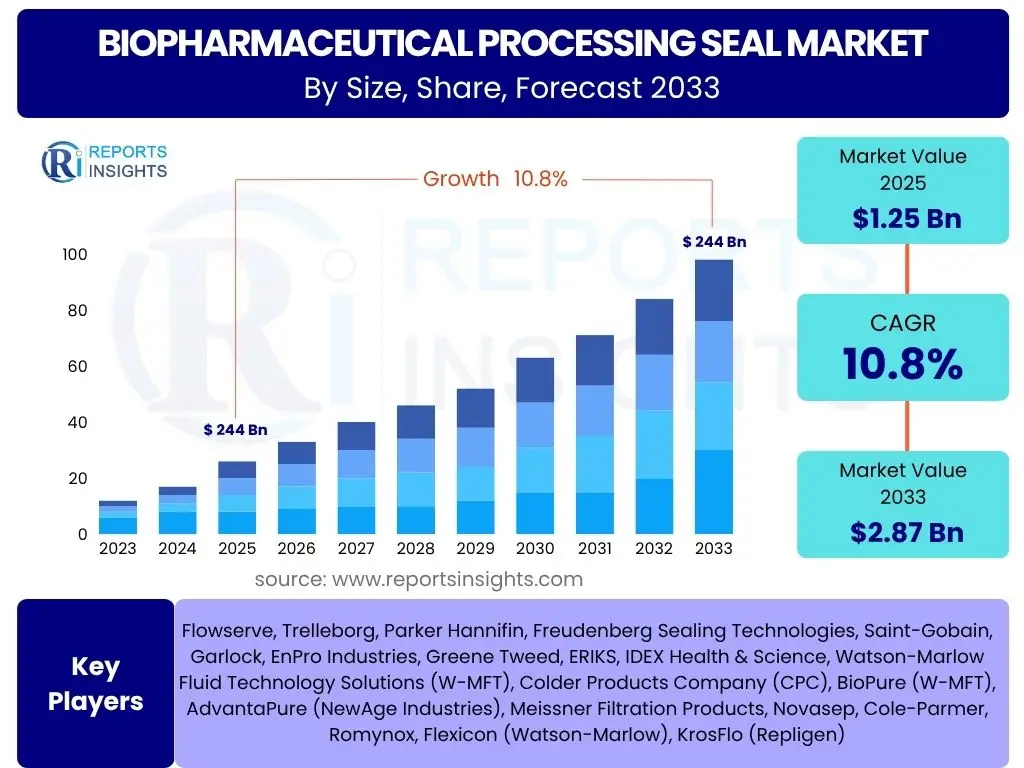
According to Reports Insights Consulting Pvt Ltd, The Biopharmaceutical Processing Seal Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 10.8% between 2025 and 2033. The market is estimated at USD 1.25 Billion in 2025 and is projected to reach USD 2.87 Billion by the end of the forecast period in 2033.
Key Biopharmaceutical Processing Seal Market Trends & Insights
Common user inquiries about the Biopharmaceutical Processing Seal market trends frequently revolve around the adoption of single-use technologies, advancements in material science, and the increasing demand for high-purity and compliant sealing solutions. Users are particularly interested in how these trends impact operational efficiency, reduce contamination risks, and align with evolving regulatory landscapes. The shift towards more flexible and disposable bioprocessing setups is a dominant theme, alongside the quest for seals capable of withstanding harsh sterilization cycles and aggressive media without compromising integrity or product quality. Furthermore, there is growing attention on sustainable practices and the lifecycle management of processing components, including seals.
Another significant area of interest concerns the integration of advanced manufacturing techniques, such as additive manufacturing, for creating custom or complex seal geometries, and the development of 'smart' seals equipped with sensors for real-time monitoring. Users are keen to understand how these innovations contribute to predictive maintenance, reduce downtime, and enhance the overall reliability of bioprocessing systems. The expansion of cell and gene therapies, which often require highly specialized and sterile processing environments, is also a key trend driving demand for seals with specific performance characteristics, prompting questions about material compatibility and extractables profiles in these novel applications.
- Growing adoption of single-use bioprocessing systems.
- Development of advanced, high-performance seal materials (e.g., perfluoroelastomers, reinforced PTFE).
- Increasing demand for aseptic and sterile connectors and seals.
- Integration of smart seals with monitoring capabilities.
- Emphasis on extractables and leachables testing and compliance.
- Customization of seals for specific bioprocessing applications.
- Sustainability initiatives influencing material selection and disposal.
AI Impact Analysis on Biopharmaceutical Processing Seal
User questions regarding the impact of Artificial Intelligence (AI) on the Biopharmaceutical Processing Seal domain often explore its potential to enhance design, manufacturing efficiency, and predictive maintenance. Users are curious about how AI algorithms can optimize seal geometries for better performance and longevity, or predict potential seal failures before they occur, thereby minimizing costly downtime in critical bioprocessing operations. There is also interest in AI's role in quality control, particularly in detecting minute defects during manufacturing that might be imperceptible to human inspection, ensuring higher product reliability and safety standards.
Furthermore, inquiries frequently touch upon AI-driven supply chain optimization for seal components, focusing on inventory management, demand forecasting, and risk mitigation. The ability of AI to analyze vast datasets related to material properties, processing conditions, and operational performance could lead to more informed decisions regarding seal selection and application, ensuring optimal compatibility and regulatory adherence. While the direct application of AI within the seal itself is nascent, its broader influence on the bioprocessing ecosystem—from R&D to manufacturing and maintenance—is anticipated to significantly improve the efficiency and reliability of sealing solutions.
- AI-driven optimization of seal design and material selection.
- Predictive maintenance for seals using AI algorithms to forecast failure.
- Enhanced quality control and defect detection in seal manufacturing.
- Supply chain optimization for seal components through AI analytics.
- Improved process parameter optimization and anomaly detection in bioprocessing systems, influencing seal performance.
Key Takeaways Biopharmaceutical Processing Seal Market Size & Forecast
Common user questions about the Biopharmaceutical Processing Seal market size and forecast reveal a keen interest in the trajectory of market growth, the primary drivers sustaining this expansion, and the critical role seals play in the rapidly evolving biopharmaceutical landscape. Users seek to understand if the market will maintain its robust growth, which specific segments are poised for the highest acceleration, and how the global regulatory environment influences market dynamics. The insights derived highlight that the market is on a strong upward trajectory, fueled by consistent innovation in biopharmaceuticals and an increasing focus on process efficiency and patient safety.
A significant takeaway is the indispensable nature of high-quality seals in preventing contamination and ensuring product integrity throughout complex bioprocessing workflows, from upstream fermentation to downstream purification and fill-finish operations. The market's resilience is also attributed to the continuous investment in biopharmaceutical R&D, particularly in novel therapies like gene and cell therapies, which demand highly specialized and reliable sealing solutions. The forecast indicates sustained growth, with opportunities for manufacturers to differentiate through superior material science, customized solutions, and adherence to stringent industry standards.
- The Biopharmaceutical Processing Seal market is poised for significant and sustained growth through 2033.
- Market expansion is primarily driven by the robust growth of the biopharmaceutical industry and increasing adoption of single-use technologies.
- High-purity and regulatory-compliant seals are critical for maintaining aseptic conditions and product integrity.
- Innovation in material science and seal design is essential for meeting evolving bioprocessing demands.
- The market presents substantial opportunities for specialized manufacturers, especially in emerging therapeutic areas.
Biopharmaceutical Processing Seal Market Drivers Analysis
The Biopharmaceutical Processing Seal market is primarily driven by the escalating demand for biologics, biosimilars, and advanced therapies such as gene and cell therapies. The global biopharmaceutical industry is experiencing unprecedented growth, fueled by an aging population, rising prevalence of chronic diseases, and significant investments in research and development. As biopharmaceutical production scales up and becomes more complex, the need for sterile, leak-proof, and highly reliable sealing solutions becomes paramount to ensure product quality, patient safety, and regulatory compliance. This inherent demand translates directly into increased consumption of specialized seals designed for bioprocessing environments.
Another pivotal driver is the accelerating adoption of single-use bioprocessing technologies. Single-use systems mitigate the risks of cross-contamination, reduce cleaning and sterilization validation efforts, and offer greater flexibility and faster turnaround times compared to traditional stainless-steel equipment. Seals are integral components of these single-use assemblies, and their disposable nature drives recurring demand. Furthermore, stringent regulatory requirements, particularly from bodies like the FDA and EMA, mandate the use of biocompatible and extractables-free materials, pushing manufacturers to innovate and develop seals that meet the highest purity and safety standards.
| Drivers | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Growing Biologics & Biosimilars Market | +3.0% | Global, particularly North America, Europe, APAC | Long-term (2025-2033) |
| Increasing Adoption of Single-Use Bioreactors & Systems | +2.5% | Global, strong in developed markets | Mid-term (2025-2029) |
| Stringent Regulatory Standards & Quality Demands | +1.8% | Global, especially highly regulated regions | Long-term (2025-2033) |
| Growth in Cell & Gene Therapy Pipelines | +1.5% | North America, Europe, emerging in APAC | Long-term (2027-2033) |
| Advancements in Seal Materials & Design | +1.0% | Global | Mid-term (2026-2031) |
Biopharmaceutical Processing Seal Market Restraints Analysis
The Biopharmaceutical Processing Seal market faces several notable restraints that could temper its growth trajectory. One significant challenge is the high cost associated with advanced, high-purity seals, particularly those made from specialized elastomers and fluoropolymers. These materials, while essential for maintaining aseptic conditions and preventing contamination, often come with a premium price tag due to their complex manufacturing processes, rigorous quality control, and specialized raw material sourcing. This elevated cost can be a barrier for smaller biopharmaceutical companies or those operating with tighter budgets, potentially leading them to seek less expensive, albeit potentially less optimal, sealing solutions.
Another restraint involves the complexities surrounding material compatibility and extractables/leachables testing. Biopharmaceutical products are highly sensitive, and any interaction with processing components, including seals, can compromise product integrity, efficacy, or safety. The extensive and time-consuming validation processes required to ensure that seals do not leach harmful substances into the drug product can delay market entry for new seal designs or materials. Furthermore, the stringent qualification and regulatory approval processes for new bioprocessing components, including seals, impose significant burdens on manufacturers, requiring substantial investment in testing and documentation, which can slow down innovation and adoption rates.
| Restraints | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| High Cost of Advanced, High-Purity Seals | -1.2% | Global, especially emerging markets | Long-term (2025-2033) |
| Stringent Material Compatibility & E&L Testing Requirements | -1.0% | Global, highly regulated regions | Long-term (2025-2033) |
| Complex Regulatory Approval & Qualification Processes | -0.8% | Global | Long-term (2025-2033) |
| Competition from Alternative Connection Technologies | -0.5% | Global | Mid-term (2025-2029) |
Biopharmaceutical Processing Seal Market Opportunities Analysis
The Biopharmaceutical Processing Seal market is brimming with opportunities, largely driven by the continuous evolution of the biopharmaceutical industry itself. The rapid expansion of novel therapeutic areas, particularly cell and gene therapies, personalized medicine, and mRNA-based vaccines, creates a demand for highly specialized and robust sealing solutions. These advanced therapies often require unique processing conditions, ultra-high purity materials, and customizable seal geometries to ensure product integrity and patient safety, opening new avenues for innovation and market penetration for seal manufacturers capable of meeting these specific demands.
Another significant opportunity lies in the continued geographical expansion of biopharmaceutical manufacturing, especially in emerging economies. Countries in Asia Pacific, Latin America, and parts of the Middle East and Africa are increasing their investments in bioprocessing infrastructure and manufacturing capabilities. This decentralization of production creates new markets for seal suppliers, who can leverage partnerships with local manufacturers and offer tailored solutions to meet regional regulatory and operational needs. Furthermore, the increasing adoption of continuous bioprocessing and modular manufacturing facilities presents opportunities for seals that offer enhanced durability and performance under sustained operational stress, driving demand for innovative and long-lasting sealing components.
| Opportunities | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Expansion of Cell & Gene Therapy Manufacturing | +2.0% | North America, Europe, APAC | Long-term (2026-2033) |
| Geographical Expansion in Emerging Biopharma Markets | +1.8% | APAC, Latin America, MEA | Mid-term (2025-2030) |
| Development of Smart Seals & IoT Integration | +1.5% | Global, technologically advanced regions | Long-term (2027-2033) |
| Focus on Sustainable & Environmentally Friendly Materials | +1.0% | Europe, North America | Mid-term (2025-2030) |
| Customization for Niche & Specialized Applications | +0.8% | Global | Long-term (2025-2033) |
Biopharmaceutical Processing Seal Market Challenges Impact Analysis
The Biopharmaceutical Processing Seal market encounters several significant challenges that necessitate constant innovation and adaptation from manufacturers. One primary challenge is ensuring complete material compatibility and minimizing extractables and leachables (E&L) to an undetectable level. Biopharmaceutical products are highly sensitive, and even minute amounts of leached compounds from seals can compromise product purity, stability, or patient safety, leading to costly recalls or regulatory non-compliance. Developing new materials that offer superior performance without contributing to E&L profiles requires extensive R&D and rigorous testing, which can be time-consuming and expensive.
Another pressing challenge is the rapid pace of technological advancements within the biopharmaceutical sector, particularly the emergence of new processing techniques and modalities. This necessitates continuous adaptation in seal design and material science to meet evolving operational parameters, such as higher pressures, varying temperatures, or compatibility with novel solvents and buffers. Maintaining consistency in quality and supply chain resilience amidst global disruptions and fluctuating raw material costs also poses a persistent challenge, requiring robust risk management strategies and diversified sourcing. Furthermore, the specialized knowledge required for designing, manufacturing, and implementing high-performance bioprocessing seals contributes to a talent gap, affecting both manufacturers and end-users.
| Challenges | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Maintaining Ultra-High Purity & Minimizing E&L | -1.5% | Global | Long-term (2025-2033) |
| Rapid Technological Evolution in Bioprocessing | -1.0% | Global | Long-term (2025-2033) |
| Supply Chain Volatility & Raw Material Costs | -0.7% | Global | Short-term (2025-2027) |
| Counterfeiting & Quality Control Issues | -0.5% | Global, particularly emerging markets | Long-term (2025-2033) |
Biopharmaceutical Processing Seal Market - Updated Report Scope
This report provides an in-depth analysis of the global Biopharmaceutical Processing Seal market, offering a comprehensive overview of market dynamics, segmentation, regional landscapes, and the competitive environment. It delves into critical market drivers, restraints, opportunities, and challenges that shape the industry's trajectory. The scope includes historical data, current market sizing, and future growth projections, alongside a detailed examination of technological advancements and their impact on market evolution. The report serves as a strategic tool for stakeholders, enabling informed decision-making and identification of key investment areas within the biopharmaceutical processing seal sector.
| Report Attributes | Report Details |
|---|---|
| Base Year | 2024 |
| Historical Year | 2019 to 2023 |
| Forecast Year | 2025 - 2033 |
| Market Size in 2025 | USD 1.25 Billion |
| Market Forecast in 2033 | USD 2.87 Billion |
| Growth Rate | 10.8% CAGR |
| Number of Pages | 250 |
| Key Trends | |
| Segments Covered | |
| Key Companies Covered | Flowserve, Trelleborg, Parker Hannifin, Freudenberg Sealing Technologies, Saint-Gobain, Garlock, EnPro Industries, Greene Tweed, ERIKS, IDEX Health & Science, Watson-Marlow Fluid Technology Solutions (W-MFT), Colder Products Company (CPC), BioPure (W-MFT), AdvantaPure (NewAge Industries), Meissner Filtration Products, Novasep, Cole-Parmer, Romynox, Flexicon (Watson-Marlow), KrosFlo (Repligen) |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Speak to Analyst | Avail customised purchase options to meet your exact research needs. Request For Analyst Or Customization |
Segmentation Analysis
The Biopharmaceutical Processing Seal market is meticulously segmented to provide a granular understanding of its diverse components and evolving dynamics. This comprehensive segmentation allows for a detailed analysis of market performance across different materials, seal types, applications, and end-use sectors, reflecting the specialized requirements of the biopharmaceutical industry. Each segment plays a crucial role in the overall market growth, driven by unique technological advancements, regulatory mandates, and operational demands, providing valuable insights for strategic planning and investment decisions.
Understanding these segments helps identify high-growth areas and niche opportunities, such as the increasing adoption of perfluoroelastomers for their superior chemical resistance, or the rising demand for custom-designed seals for advanced therapeutic modalities. The application-based segmentation highlights the critical role seals play across the entire bioprocessing workflow, from upstream fermentation to downstream purification and final fill-finish, emphasizing the need for robust and reliable solutions at every stage. This detailed breakdown ensures that market participants can accurately assess their competitive landscape and tailor their product offerings to specific industry needs.
- By Material:
- Elastomers (EPDM, FKM, Silicone, NBR, Others)
- Fluoropolymers (PTFE, PFA, FEP)
- Perfluoroelastomers (FFKM)
- Others (UHMW-PE, Stainless Steel)
- By Type:
- O-Rings
- Gaskets
- Diaphragms
- Custom Seals
- Lip Seals
- Shaft Seals
- By Application:
- Fermentation
- Filtration
- Chromatography
- Mixing
- Centrifugation
- Fill-Finish
- Sterilization
- By End-Use:
- Biopharmaceutical Manufacturers
- Contract Manufacturing Organizations (CMOs) / Contract Development and Manufacturing Organizations (CDMOs)
- Research & Development Institutions
- Academic & Government Research Labs
Regional Highlights
The Biopharmaceutical Processing Seal market exhibits distinct regional dynamics, influenced by varying levels of biopharmaceutical R&D, manufacturing capabilities, and regulatory frameworks. North America, particularly the United States, represents a dominant market share due to its robust biopharmaceutical industry, significant investments in R&D, and early adoption of advanced bioprocessing technologies, including single-use systems. The presence of numerous leading biopharmaceutical companies and a strong regulatory environment drives consistent demand for high-quality, compliant seals. The region continues to be a hub for innovation in cell and gene therapies, further bolstering its market position.
Europe also holds a substantial share in the biopharmaceutical processing seal market, characterized by its well-established pharmaceutical sector, strong emphasis on quality and regulatory compliance, and a growing number of Contract Manufacturing and Development Organizations (CMOs/CDMOs). Countries like Germany, the UK, France, and Switzerland are key contributors, driven by extensive research activities and a focus on sustainable bioprocessing. Asia Pacific is emerging as the fastest-growing region, propelled by increasing healthcare expenditure, rising prevalence of chronic diseases, government support for the biopharmaceutical sector, and the expansion of manufacturing capacities in countries like China, India, and South Korea. Latin America, the Middle East, and Africa (MEA) are also showing promising growth, albeit from a smaller base, as these regions enhance their biopharmaceutical production capabilities and strive for self-sufficiency in drug manufacturing.
- North America: Dominates the market due to robust biopharma R&D, high adoption of single-use technologies, and stringent regulatory standards. Focus on advanced therapies (cell and gene therapy).
- Europe: Significant market share with strong regulatory frameworks, established biopharmaceutical manufacturing base, and increasing focus on bioprocessing innovation and sustainability.
- Asia Pacific (APAC): Fastest-growing region driven by expanding biomanufacturing capabilities, rising healthcare investments, increasing prevalence of chronic diseases, and emerging contract manufacturing hubs.
- Latin America: Growing market spurred by increasing healthcare investments and local production initiatives, albeit with varying regulatory landscapes.
- Middle East and Africa (MEA): Emerging market with increasing government focus on developing local pharmaceutical and biopharmaceutical industries, leading to gradual adoption of advanced processing components.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Biopharmaceutical Processing Seal Market.- Flowserve
- Trelleborg
- Parker Hannifin
- Freudenberg Sealing Technologies
- Saint-Gobain
- Garlock
- EnPro Industries
- Greene Tweed
- ERIKS
- IDEX Health & Science
- Watson-Marlow Fluid Technology Solutions (W-MFT)
- Colder Products Company (CPC)
- BioPure (part of W-MFT)
- AdvantaPure (part of NewAge Industries)
- Meissner Filtration Products
- Novasep
- Cole-Parmer
- Romynox
- Flexicon (Watson-Marlow)
- KrosFlo (Repligen)
Frequently Asked Questions
What are the primary types of seals used in biopharmaceutical processing?
The primary types of seals include O-rings, gaskets, diaphragms, and custom-engineered seals. These are crucial for maintaining sterility and preventing contamination across various bioprocessing equipment like bioreactors, filtration systems, and chromatography columns.
Why is there a growing trend towards single-use seals in bioprocessing?
Single-use seals are gaining traction due to their ability to eliminate the need for cleaning and sterilization validation, reduce the risk of cross-contamination, enhance operational flexibility, and lower overall turnaround times, contributing to cost efficiency in manufacturing.
How do regulatory standards impact the selection of biopharmaceutical processing seals?
Stringent regulatory bodies such as the FDA and EMA mandate that seals meet high standards for biocompatibility, material purity, and minimal extractables and leachables (E&L). Compliance with these regulations is critical to ensure product safety and efficacy, driving the demand for specialized, certified sealing solutions.
What are the key materials used for biopharmaceutical processing seals?
Key materials include elastomers (like EPDM, FKM, Silicone), fluoropolymers (such as PTFE, PFA), and high-performance perfluoroelastomers (FFKM). The choice of material depends on factors such as chemical compatibility, temperature resistance, sterilization methods, and extractables profiles.
What are the future growth prospects for the Biopharmaceutical Processing Seal market?
The market is projected for robust growth, driven by the expanding biologics and biosimilars market, increasing adoption of advanced therapies like cell and gene therapies, and continued innovation in single-use bioprocessing technologies. Emerging markets are also expected to contribute significantly to this growth.

