Single Use Medical Device Reprocessing Market
Single Use Medical Device Reprocessing Market Size, Scope, Growth, Trends and By Segmentation Types, Applications, Regional Analysis and Industry Forecast (2025-2033)
Report ID : RI_703873 | Last Updated : August 05, 2025 |
Format : ![]()
![]()
![]()
![]()
Single Use Medical Device Reprocessing Market Size
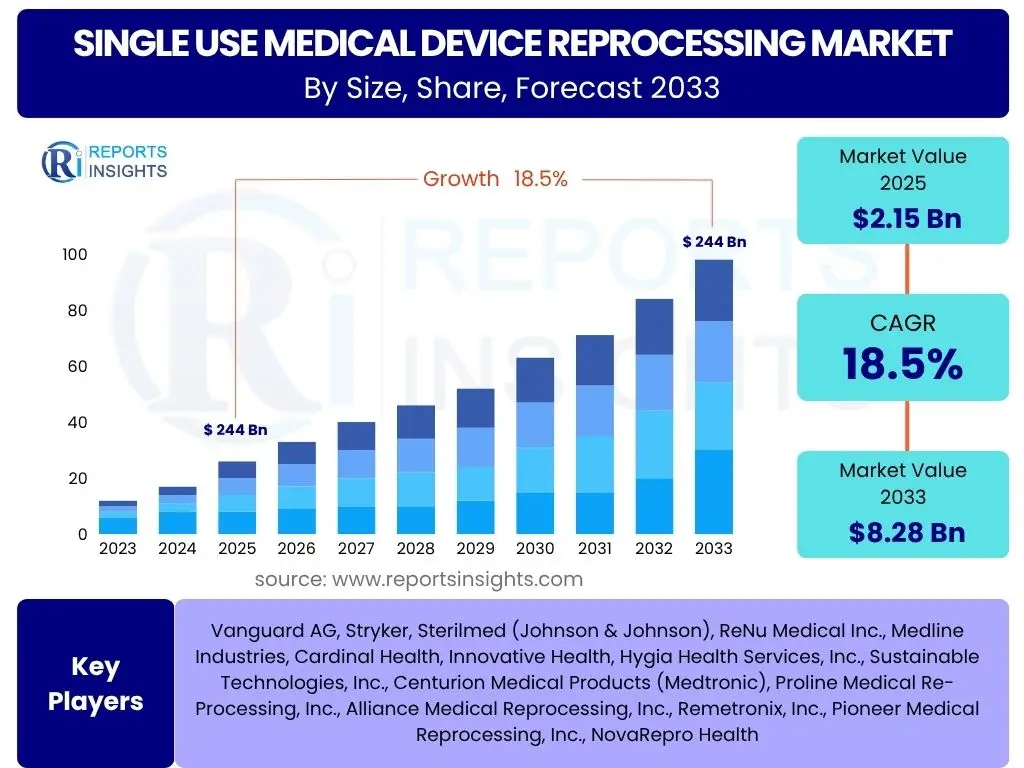
According to Reports Insights Consulting Pvt Ltd, The Single Use Medical Device Reprocessing Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 18.5% between 2025 and 2033. The market is estimated at USD 2.15 Billion in 2025 and is projected to reach USD 8.28 Billion by the end of the forecast period in 2033.
Key Single Use Medical Device Reprocessing Market Trends & Insights
The Single Use Medical Device Reprocessing (SUMDR) market is experiencing dynamic shifts, driven by a confluence of economic pressures, environmental consciousness, and technological advancements. Healthcare providers are increasingly scrutinizing operational costs, making reprocessing a compelling strategy for significant savings without compromising patient safety or quality of care. This focus on cost efficiency is a primary driver, particularly in developed healthcare systems facing budget constraints.
Furthermore, the growing emphasis on sustainability and waste reduction within the healthcare sector is significantly influencing market trends. Reprocessing single-use devices aligns directly with green initiatives, helping hospitals reduce their environmental footprint by diverting medical waste from landfills. This dual benefit of cost savings and environmental responsibility is fostering broader acceptance and adoption of reprocessing practices globally. Regulatory landscapes are also evolving, with some regions establishing clearer guidelines that support and even encourage safe and effective reprocessing, further bolstering market growth.
- Increasing pressure on healthcare providers to reduce operational costs.
- Growing emphasis on environmental sustainability and waste reduction in healthcare.
- Technological advancements in reprocessing techniques ensuring higher efficacy and safety.
- Favorable or clarifying regulatory frameworks in key regions supporting reprocessing.
- Rising awareness and acceptance among healthcare professionals regarding the safety and benefits of reprocessed devices.
AI Impact Analysis on Single Use Medical Device Reprocessing
Artificial intelligence (AI) is poised to significantly transform the Single Use Medical Device Reprocessing (SUMDR) market by enhancing efficiency, precision, and quality control throughout the reprocessing cycle. Users frequently inquire about AI's potential to automate complex inspection processes, improve data analytics for predictive maintenance of reprocessing equipment, and optimize sterilization protocols. The primary expectation is that AI can minimize human error, reduce reprocessing times, and ensure a higher standard of device readiness, directly impacting patient safety and operational throughput.
Concerns often revolve around the initial investment required for AI integration, the complexity of validating AI algorithms in highly regulated medical environments, and the need for skilled personnel to manage these advanced systems. However, the overarching theme is optimism regarding AI's ability to provide a competitive edge through enhanced traceability, real-time quality assurance, and more robust compliance management. AI's capacity to process vast amounts of data can also lead to improved understanding of device lifespan and reprocessing limits, contributing to more informed decision-making within reprocessing facilities.
- Enhanced quality control and inspection through AI-powered visual recognition systems.
- Optimization of reprocessing workflows and cycle times using predictive analytics.
- Improved traceability and inventory management of reprocessed devices.
- Automated data analysis for compliance reporting and process validation.
- Predictive maintenance for reprocessing equipment, reducing downtime and operational costs.
Key Takeaways Single Use Medical Device Reprocessing Market Size & Forecast
The Single Use Medical Device Reprocessing (SUMDR) market is on a robust growth trajectory, driven by an imperative to optimize healthcare expenditures and mitigate environmental impact. Key user questions frequently center on the economic viability of reprocessing, the safety assurance of reprocessed devices, and the future regulatory landscape. The market's significant Compound Annual Growth Rate (CAGR) indicates a strong and sustained shift towards sustainable and cost-effective healthcare solutions, making reprocessing an increasingly integral part of hospital operations worldwide.
A crucial insight is that continued technological innovation in cleaning, disinfection, and sterilization technologies, combined with stringent quality assurance protocols, will be paramount to maintaining market confidence and expanding reprocessing capabilities to a wider array of medical devices. Furthermore, the market's expansion is expected to be uneven across geographies, with North America and Europe leading due to established regulatory frameworks and high awareness, while emerging economies present substantial long-term growth opportunities as their healthcare infrastructure develops and cost-saving pressures intensify. Stakeholders should focus on demonstrating quantifiable safety and efficacy data to overcome lingering perceptions and capitalize on this growing market.
- The market exhibits strong growth potential, primarily driven by cost-saving mandates and environmental sustainability goals in healthcare.
- Technological advancements in reprocessing methods are crucial for expanding the range and safety of reprocessed devices.
- North America and Europe currently dominate due to established regulations and high adoption rates, but Asia Pacific offers significant future growth.
- Addressing perceptions regarding device safety and efficacy through robust data and transparent practices is key for market penetration.
- The economic benefits for healthcare providers, coupled with reduced medical waste, position reprocessing as a critical component of modern healthcare delivery.
Single Use Medical Device Reprocessing Market Drivers Analysis
The expansion of the Single Use Medical Device Reprocessing (SUMDR) market is primarily propelled by the persistent need for cost containment within healthcare systems globally. As healthcare expenditures continue to rise, hospitals and ambulatory surgical centers are actively seeking strategies to reduce operational costs without compromising patient care quality. Reprocessing offers a direct pathway to significant savings on medical device procurement, making it an attractive option for budget-conscious facilities.
Beyond economic considerations, a growing global awareness of environmental sustainability is also a powerful driver. Healthcare facilities generate substantial amounts of waste, and single-use medical devices contribute significantly to this burden. Reprocessing helps reduce the volume of medical waste sent to landfills, aligning with corporate social responsibility initiatives and contributing to a greener healthcare industry. Additionally, the increasing volume of surgical procedures performed worldwide, coupled with the rising prevalence of chronic diseases, necessitates a greater supply of medical devices, for which reprocessing offers a sustainable and efficient solution.
| Drivers | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Healthcare Cost Containment | +5.0% | Global, particularly North America, Europe | Short to Medium Term (2025-2029) |
| Growing Emphasis on Environmental Sustainability | +4.5% | North America, Europe, parts of Asia Pacific | Medium to Long Term (2027-2033) |
| Increasing Volume of Surgical Procedures | +4.0% | Global, especially emerging economies | Short to Medium Term (2025-2030) |
| Favorable Regulatory Support & Clarity | +3.5% | North America, select European countries | Medium Term (2026-2031) |
| Technological Advancements in Reprocessing | +3.0% | Global | Medium to Long Term (2027-2033) |
Single Use Medical Device Reprocessing Market Restraints Analysis
Despite the compelling drivers, the Single Use Medical Device Reprocessing (SUMDR) market faces notable restraints, primarily centered around lingering perceptions and regulatory complexities. A significant barrier is the persistent apprehension among some healthcare professionals and the public regarding the safety and efficacy of reprocessed devices. Concerns about potential infection risks or compromised device functionality, although often unsubstantiated by clinical data for properly reprocessed devices, can impede broader adoption and create market resistance.
Furthermore, the varied and sometimes ambiguous regulatory landscape across different countries poses a significant challenge. While regions like North America have clear guidelines, many other parts of the world lack comprehensive regulations or have outright bans on reprocessing certain device types, creating market fragmentation and limiting global expansion. The initial capital investment required for establishing or upgrading reprocessing facilities, coupled with the need for specialized equipment and highly trained personnel, can also act as a deterrent, especially for smaller healthcare institutions or those in less developed regions. Overcoming these restraints requires extensive education, transparent reporting of safety data, and harmonization of international regulations.
| Restraints | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Perception and Safety Concerns | -3.5% | Global | Long Term (2025-2033) |
| Varying & Complex Regulatory Landscape | -3.0% | Global, especially Asia Pacific, Latin America | Long Term (2025-2033) |
| High Initial Investment for Reprocessing Infrastructure | -2.5% | Emerging Economies, Smaller Facilities | Short to Medium Term (2025-2030) |
| OEM Resistance and Business Models | -2.0% | Global | Long Term (2025-2033) |
| Risk of Cross-Contamination & Efficacy Loss | -1.5% | Global | Short Term (2025-2027) |
Single Use Medical Device Reprocessing Market Opportunities Analysis
Significant opportunities exist within the Single Use Medical Device Reprocessing (SUMDR) market, particularly in expanding into untapped geographical regions and diversifying the types of devices that can be safely reprocessed. Emerging economies, driven by rapidly expanding healthcare infrastructure and a strong need for cost-effective solutions, represent fertile ground for market penetration. These regions often face significant budgetary constraints, making reprocessing an even more attractive proposition than in developed markets, provided regulatory frameworks can be established and adhered to.
Furthermore, continuous innovation in reprocessing technologies opens doors to handling a broader spectrum of complex medical devices that were previously deemed unreprocessable. Advancements in cleaning agents, sterilization methods, and advanced inspection techniques are enabling the safe reprocessing of more intricate devices, thereby expanding the addressable market. Strategic partnerships between reprocessing companies, device manufacturers, and healthcare providers can also unlock new opportunities, facilitating knowledge transfer, improving standardization, and fostering greater trust in reprocessed products. The shift towards value-based healthcare models globally also aligns well with reprocessing, as it promotes efficiency and cost-effectiveness while maintaining quality outcomes.
| Opportunities | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Expansion into Emerging Markets | +4.0% | Asia Pacific, Latin America, MEA | Medium to Long Term (2028-2033) |
| Technological Advancements in Reprocessing | +3.5% | Global | Medium to Long Term (2027-2033) |
| Broadening Device Types for Reprocessing | +3.0% | Global | Medium Term (2026-2031) |
| Strategic Partnerships & Collaborations | +2.5% | Global | Short to Medium Term (2025-2029) |
| Value-Based Healthcare Models | +2.0% | North America, Europe | Medium Term (2026-2031) |
Single Use Medical Device Reprocessing Market Challenges Impact Analysis
The Single Use Medical Device Reprocessing (SUMDR) market faces several critical challenges that could impede its growth and widespread acceptance. One significant hurdle is the ongoing battle against negative perceptions, often fueled by original equipment manufacturers (OEMs) who have a vested interest in promoting the sale of new devices. This can manifest as skepticism among healthcare professionals or the public regarding the safety and quality of reprocessed devices, necessitating continuous educational efforts and transparent data sharing by reprocessing companies.
Another major challenge is navigating the fragmented and often inconsistent regulatory frameworks across different countries and regions. A lack of uniform standards for reprocessing can create compliance complexities for market players and may deter new entrants. Ensuring consistent quality control and validation throughout the entire reprocessing cycle for a diverse range of devices is also a constant operational challenge, requiring robust systems and highly skilled personnel to mitigate any risks. Furthermore, managing the reverse logistics and supply chain for collecting, transporting, and delivering reprocessed devices efficiently adds another layer of complexity to operations.
| Challenges | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Overcoming Negative Perceptions & OEM Resistance | -3.0% | Global | Long Term (2025-2033) |
| Regulatory & Compliance Complexity | -2.5% | Global, especially highly regulated markets | Long Term (2025-2033) |
| Ensuring Consistent Quality & Safety Validation | -2.0% | Global | Short to Medium Term (2025-2029) |
| Logistics & Supply Chain Management | -1.5% | Global | Short Term (2025-2027) |
| Limited Reprocessing Cycles for Certain Devices | -1.0% | Global | Medium Term (2026-2031) |
Single Use Medical Device Reprocessing Market - Updated Report Scope
This report provides an in-depth analysis of the Single Use Medical Device Reprocessing market, encompassing historical data, current market dynamics, and future projections. It delivers comprehensive insights into market size, growth drivers, restraints, opportunities, and challenges affecting the industry. The study covers key market segments by device type, reprocessing process, and end-user, along with a detailed regional analysis, offering a holistic view of the market landscape and competitive environment. The report also includes an impact analysis of emerging technologies such as Artificial Intelligence on the reprocessing sector.
| Report Attributes | Report Details |
|---|---|
| Base Year | 2024 |
| Historical Year | 2019 to 2023 |
| Forecast Year | 2025 - 2033 |
| Market Size in 2025 | USD 2.15 Billion |
| Market Forecast in 2033 | USD 8.28 Billion |
| Growth Rate | 18.5% |
| Number of Pages | 257 |
| Key Trends |
|
| Segments Covered |
|
| Key Companies Covered | Vanguard AG, Stryker, Sterilmed (Johnson & Johnson), ReNu Medical Inc., Medline Industries, Cardinal Health, Innovative Health, Hygia Health Services, Inc., Sustainable Technologies, Inc., Centurion Medical Products (Medtronic), Proline Medical Re-Processing, Inc., Alliance Medical Reprocessing, Inc., Remetronix, Inc., Pioneer Medical Reprocessing, Inc., NovaRepro Health |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Speak to Analyst | Avail customised purchase options to meet your exact research needs. Request For Analyst Or Customization |
Segmentation Analysis
The Single Use Medical Device Reprocessing market is comprehensively segmented to provide a granular understanding of its diverse components and drivers. This segmentation allows for targeted analysis of growth opportunities and challenges within specific device categories, reprocessing techniques, and end-user environments. Such detailed categorization helps stakeholders identify niche markets and tailor their strategies to address the unique needs and regulatory considerations of each segment.
Understanding these segments is critical for market participants, enabling them to optimize product development, marketing efforts, and distribution channels. For instance, the market dynamics and technological requirements for reprocessing a cardiovascular catheter differ significantly from those for an orthopedic drill. Similarly, the operational scale and specific needs of a large hospital vary greatly from those of a specialized ambulatory surgical center, influencing the type and volume of reprocessing services demanded.
- By Device Type: This segment includes Cardiovascular Devices (e.g., Electrophysiology Catheters, Diagnostic Catheters, Guidewires), Gastroenterology Devices (e.g., Biopsy Forceps, Endoscopic Devices), Orthopedic Devices (e.g., Drills, Blades, Arthroscopic Shavers), General Surgery Devices (e.g., Laparoscopic Instruments, Harmonic Scalpels), and Other Devices (e.g., Urology, Neurology, Respiratory Devices).
- By Process: This segment covers the various stages involved in reprocessing, including Cleaning, Disinfection, Sterilization, Testing & Validation, and Packaging.
- By End User: This segment categorizes the market based on the primary users of reprocessed devices, such as Hospitals, Ambulatory Surgical Centers (ASCs), Specialty Clinics, and Other Healthcare Facilities.
Regional Highlights
- North America: This region currently holds the largest share of the Single Use Medical Device Reprocessing market, primarily driven by stringent healthcare cost-reduction mandates, well-established regulatory frameworks from bodies like the FDA that support reprocessing, and a high level of awareness among healthcare providers. The presence of key market players and advanced reprocessing infrastructure further solidifies its dominant position.
- Europe: Europe represents a significant market, characterized by evolving regulatory landscapes and a strong emphasis on environmental sustainability. While some European countries have stringent regulations or outright bans on certain reprocessed devices, others are increasingly adopting reprocessing practices to manage healthcare costs. Germany, the UK, and France are notable contributors to market growth in this region.
- Asia Pacific (APAC): The APAC region is projected to exhibit the fastest growth over the forecast period. This growth is attributed to the rapidly expanding healthcare infrastructure, increasing healthcare expenditure, a large patient pool, and a growing recognition of the economic and environmental benefits of reprocessing. Countries like China, India, and Japan are emerging as key markets, though regulatory clarity remains a crucial factor for widespread adoption.
- Latin America: This region is experiencing steady growth in the reprocessing market, driven by escalating healthcare costs and a rising demand for affordable medical solutions. Brazil and Mexico are leading the adoption of reprocessing, albeit with varying degrees of regulatory maturity.
- Middle East and Africa (MEA): The MEA market is still in its nascent stages but is expected to witness gradual growth as healthcare facilities seek cost-effective solutions and sustainable practices. Increased investment in healthcare infrastructure and rising awareness about reprocessing benefits will drive adoption in countries like Saudi Arabia, UAE, and South Africa.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Single Use Medical Device Reprocessing Market.- Vanguard AG
- Stryker
- Sterilmed (Johnson & Johnson)
- ReNu Medical Inc.
- Medline Industries
- Cardinal Health
- Innovative Health
- Hygia Health Services, Inc.
- Sustainable Technologies, Inc.
- Centurion Medical Products (Medtronic)
- Proline Medical Re-Processing, Inc.
- Alliance Medical Reprocessing, Inc.
- Remetronix, Inc.
- Pioneer Medical Reprocessing, Inc.
- NovaRepro Health
- SureTek Medical
- Medical Device Reprocessing International (MDRI)
- BioMed Reprocessing Solutions
- Global Medical Reprocessing Services
- Apex Medical Reprocessing
Frequently Asked Questions
What is Single Use Medical Device Reprocessing?
Single Use Medical Device Reprocessing (SUMDR) is the process of cleaning, disinfecting or sterilizing, testing, and packaging a medical device that was originally labeled by its manufacturer for single use. This process enables the device to be safely reused on another patient, extending its lifecycle and reducing waste.
Is reprocessing single-use medical devices safe?
Yes, when performed by qualified and regulated reprocessors, reprocessing of single-use medical devices is considered safe. Reprocessed devices must meet the same safety and effectiveness standards as new devices, undergoing rigorous cleaning, disinfection/sterilization, and quality assurance testing before being returned to circulation.
Which types of medical devices can be reprocessed?
A wide range of single-use medical devices can be reprocessed, typically those that do not become contaminated with human tissue or fluids in a way that prevents effective cleaning and sterilization. Common examples include certain cardiovascular catheters, endoscopic biopsy forceps, laparoscopic instruments, and orthopedic drills. The ability to reprocess depends on the device's design, material, and regulatory approval.
What are the primary benefits of reprocessing single-use medical devices?
The main benefits include significant cost savings for healthcare providers, often ranging from 25% to 50% compared to purchasing new devices. Additionally, it contributes to environmental sustainability by reducing medical waste sent to landfills and lowers the carbon footprint of healthcare operations.
What is the regulatory landscape for single-use medical device reprocessing?
The regulatory landscape for SUMDR varies significantly by region. In the United States, the FDA regulates reprocessors as original manufacturers, requiring them to meet stringent quality and safety standards. Europe is moving towards more harmonized regulations under the MDR, while other regions may have less developed or explicit guidelines, or outright bans on reprocessing certain devices.

