Pregnancy Rapid Test Kit Market
Pregnancy Rapid Test Kit Market Size, Scope, Growth, Trends and By Segmentation Types, Applications, Regional Analysis and Industry Forecast (2025-2033)
Report ID : RI_705164 | Last Updated : August 11, 2025 |
Format : ![]()
![]()
![]()
![]()
Pregnancy Rapid Test Kit Market Size
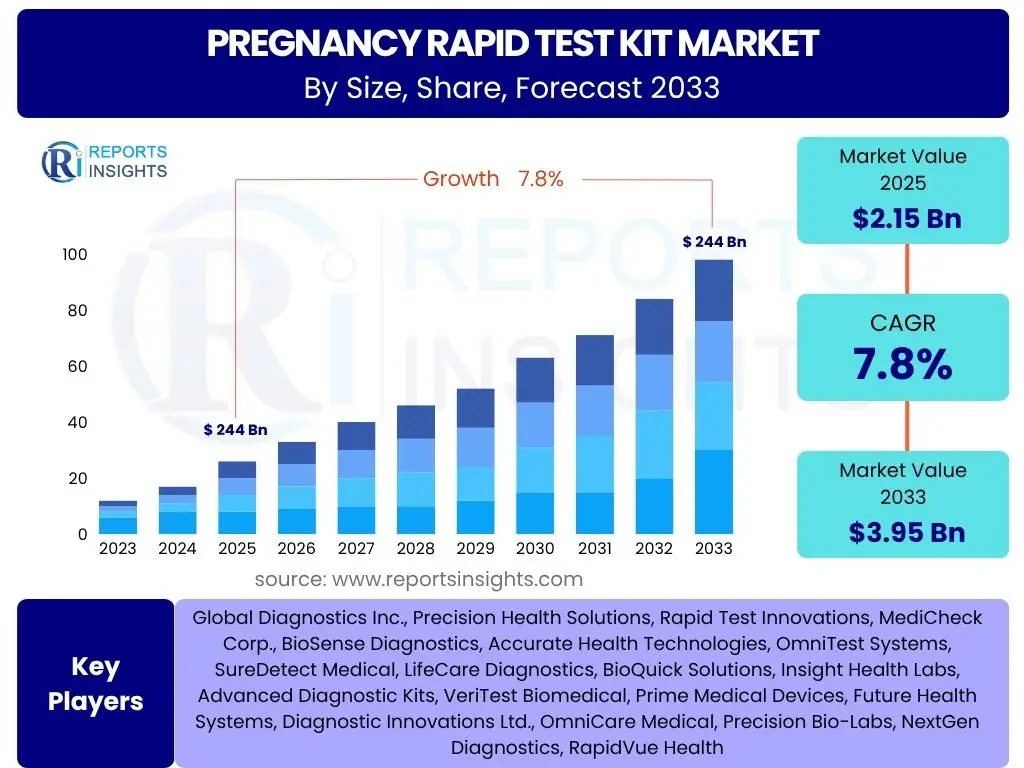
According to Reports Insights Consulting Pvt Ltd, The Pregnancy Rapid Test Kit Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 7.8% between 2025 and 2033. The market is estimated at USD 2.15 Billion in 2025 and is projected to reach USD 3.95 Billion by the end of the forecast period in 2033. This growth trajectory is indicative of increasing global awareness regarding reproductive health, coupled with a rising demand for convenient and accurate diagnostic solutions for early pregnancy detection.
The expansion of the market is primarily driven by technological advancements that enhance the reliability and ease of use of these kits. Furthermore, shifts in consumer preferences towards at-home testing, spurred by factors like privacy, accessibility, and time efficiency, significantly contribute to the market's robust expansion. The continuous innovation in rapid diagnostic technologies, including improvements in sensitivity and specificity, ensures that these kits remain a vital component of personal healthcare management.
Key Pregnancy Rapid Test Kit Market Trends & Insights
The Pregnancy Rapid Test Kit market is currently witnessing a transformative phase driven by several key trends, reflecting evolving consumer needs and technological advancements. Users frequently inquire about the integration of digital health, the quest for earlier detection capabilities, and the move towards more comprehensive diagnostic solutions. These inquiries highlight a collective desire for enhanced convenience, accuracy, and actionable insights beyond a simple positive or negative result. The market is responding by developing kits that offer greater sophistication and connectivity, aligning with broader trends in personalized and proactive healthcare.
A significant trend involves the development of multi-parameter tests that can detect not only pregnancy but also provide insights into fertility windows or potential complications. This move transcends the traditional binary result, offering users a more holistic understanding of their reproductive health. Moreover, sustainability has emerged as a crucial consideration, with a growing demand for eco-friendly and biodegradable test kits, reflecting increasing consumer environmental consciousness. The industry is actively exploring innovative materials and packaging solutions to address this demand, aiming to reduce the environmental footprint of these widely used diagnostic tools.
- Digital Integration: Emergence of smart pregnancy tests that connect with mobile applications for result tracking, cycle predictions, and personalized health advice.
- Earlier Detection Capabilities: Development of ultra-sensitive tests capable of detecting pregnancy even before a missed period, offering enhanced peace of mind.
- Multi-Parameter Tests: Introduction of kits that offer insights beyond just pregnancy, such as ovulation tracking or fertility indicators, providing a comprehensive view of reproductive health.
- At-Home Convenience and Privacy: Persistent demand for discrete, easy-to-use tests that empower individuals to manage their reproductive health privately and on their own terms.
- Sustainability Focus: Growing emphasis on environmentally friendly materials and packaging for test kits, addressing consumer demand for eco-conscious products.
AI Impact Analysis on Pregnancy Rapid Test Kit
User queries regarding the impact of Artificial Intelligence (AI) on Pregnancy Rapid Test Kits frequently revolve around how AI can enhance accuracy, provide personalized insights, and integrate with broader digital health ecosystems. There is significant interest in AI's potential to interpret subtle test line variations, reduce user error, and offer predictive analytics related to fertility and early pregnancy health. Consumers are keen to understand if AI can make at-home testing more reliable and transform a simple diagnostic tool into a comprehensive health companion.
AI's influence in this domain extends beyond mere result interpretation to include advanced data analytics that can track menstrual cycles, predict ovulation, and even flag potential concerns by analyzing user-inputted symptoms alongside test results. This capability positions AI to enable a more proactive approach to reproductive health management. While concerns about data privacy and the ethical implications of AI in personal health are present, the overwhelming expectation is that AI will significantly elevate the utility and sophistication of pregnancy rapid test kits, making them an integral part of smart health monitoring systems.
- Enhanced Accuracy and Interpretation: AI algorithms can analyze test strip images, potentially improving accuracy by objectively interpreting faint lines or ambiguous results, thereby reducing user error.
- Predictive Analytics: Integration of AI enables predictive capabilities by analyzing user data, menstrual cycles, and test results to forecast ovulation, fertility windows, and potential pregnancy outcomes.
- Smart Device Integration: AI facilitates seamless connectivity between rapid test kits and smart devices or mobile applications, providing users with personalized insights, historical data tracking, and educational content.
- Personalized User Experience: AI can tailor recommendations and information based on individual user profiles, leading to a more customized and supportive experience throughout their reproductive health journey.
- Data-Driven Insights for R&D: Aggregated, anonymized data from AI-enabled tests can provide valuable insights for manufacturers, driving research and development of more effective and targeted diagnostic solutions.
Key Takeaways Pregnancy Rapid Test Kit Market Size & Forecast
The Pregnancy Rapid Test Kit market is poised for substantial growth, driven by increasing health awareness, a preference for convenient home-based testing, and continuous technological advancements. Common user questions about key takeaways often center on the market's resilience, the impact of innovation on product utility, and the sustained demand for accessible diagnostic solutions. Insights reveal that while accuracy remains paramount, factors such as ease of use, speed of results, and integration with digital health platforms are increasingly influencing consumer choices and market expansion.
A significant takeaway is the market's evolving landscape, where basic diagnostic functionality is being augmented by value-added features, including digital connectivity and more informative results. The forecast indicates sustained investment in research and development to enhance test sensitivity and specificity, allowing for earlier and more reliable detection. This trajectory underscores a broader shift towards empowering individuals with immediate, private, and reliable tools for managing their reproductive health, contributing to improved healthcare outcomes globally.
- Robust Growth Trajectory: The market is projected to experience strong growth, reaching nearly USD 4 Billion by 2033, indicating sustained demand and market expansion.
- Technology-Driven Expansion: Continuous innovation in test sensitivity, digital integration, and AI-powered analytics is a primary catalyst for market growth.
- Increasing Consumer Empowerment: The shift towards convenient, at-home testing empowers individuals to take proactive control of their reproductive health decisions.
- Diversification of Product Offerings: Beyond basic detection, the market is moving towards multi-functional kits that offer broader reproductive health insights.
- Market Resilience: The essential nature of pregnancy detection ensures the market's stability and growth, even amidst broader economic fluctuations.
Pregnancy Rapid Test Kit Market Drivers Analysis
The Pregnancy Rapid Test Kit market is propelled by a confluence of factors that underscore a global shift towards accessible and immediate diagnostic solutions. A primary driver is the increasing global awareness and emphasis on reproductive health, leading more individuals to seek timely and private methods for pregnancy confirmation. This heightened health consciousness is augmented by rising disposable incomes in many regions, enabling broader access to and adoption of over-the-counter diagnostic tools. Furthermore, the pervasive trend towards self-care and home-based health management continues to bolster demand, as these kits offer unparalleled convenience and discretion.
Technological advancements play a crucial role, as ongoing innovations lead to more accurate, sensitive, and user-friendly test kits. Improvements in lateral flow immunoassay technology, for instance, allow for earlier detection and clearer results, enhancing consumer trust and satisfaction. The expanding global population, coupled with changing lifestyles and sexual health patterns, further contributes to a consistent demand for rapid pregnancy testing. Government initiatives and public health campaigns promoting early antenatal care also indirectly drive market growth by emphasizing the importance of timely pregnancy detection.
| Drivers | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Increasing global awareness regarding reproductive health | +1.5% | Global | Long Term (2025-2033) |
| Rising demand for home-based diagnostic solutions for convenience and privacy | +1.2% | North America, Europe, Asia Pacific | Medium to Long Term (2025-2033) |
| Technological advancements leading to enhanced accuracy and earlier detection | +1.0% | Global | Short to Medium Term (2025-2030) |
| Growing disposable income and accessibility of OTC products | +0.8% | Emerging Economies (Asia Pacific, Latin America, MEA) | Long Term (2027-2033) |
| Increasing prevalence of unplanned pregnancies worldwide | +0.7% | Global | Short to Medium Term (2025-2030) |
Pregnancy Rapid Test Kit Market Restraints Analysis
Despite robust growth, the Pregnancy Rapid Test Kit market faces certain restraints that could temper its expansion. One significant challenge is the ongoing perception regarding the accuracy and reliability of rapid tests compared to clinical laboratory methods. While rapid tests have improved significantly, some consumers or healthcare professionals may still prefer laboratory-based blood tests for definitive confirmation, particularly in critical scenarios. This perception can limit broader adoption, especially in regions with well-established clinical diagnostic infrastructures. Furthermore, potential user error in following instructions or interpreting results can lead to false positives or negatives, impacting user trust and potentially requiring subsequent professional confirmation.
Regulatory hurdles also pose a restraint, particularly in highly regulated markets where stringent approval processes and post-market surveillance requirements can delay product launches and increase development costs. This can create barriers to entry for new innovations or smaller manufacturers. Additionally, the availability of alternative, sometimes lower-cost, methods or competing diagnostic services can introduce price pressures on rapid test kit manufacturers. Concerns about the environmental impact of plastic waste from single-use kits are also growing, leading to calls for more sustainable options which might entail higher production costs or slower adoption cycles for eco-friendly alternatives.
| Restraints | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Perception of limited accuracy compared to clinical laboratory tests | -0.5% | Global | Medium Term (2025-2030) |
| Stringent regulatory approval processes and compliance costs | -0.4% | North America, Europe | Long Term (2025-2033) |
| Potential for user error in test administration and result interpretation | -0.3% | Global | Continuous |
| Growing environmental concerns regarding plastic waste from disposable kits | -0.2% | Developed Regions (North America, Europe) | Long Term (2028-2033) |
Pregnancy Rapid Test Kit Market Opportunities Analysis
The Pregnancy Rapid Test Kit market is replete with significant opportunities driven by technological advancements, evolving consumer expectations, and expanding healthcare access. One prominent opportunity lies in the integration of these tests with digital health platforms and mobile applications. Smart kits that connect to smartphones can offer personalized insights, track fertility cycles, and provide educational resources, transforming a simple diagnostic tool into a comprehensive reproductive health management system. This digital convergence appeals to tech-savvy consumers and facilitates a more proactive approach to health.
Another substantial opportunity resides in the development of highly sensitive and multi-parameter tests. Kits capable of detecting pregnancy at even earlier stages or simultaneously assessing other fertility markers (e.g., ovulation, hormonal imbalances) can cater to a broader range of consumer needs, offering enhanced value. Furthermore, underserved emerging economies, with their burgeoning populations and improving healthcare infrastructures, present lucrative avenues for market expansion. Introducing affordable and accessible rapid tests in these regions can significantly contribute to public health initiatives. The increasing demand for sustainable and eco-friendly products also opens doors for manufacturers to innovate with biodegradable materials and reduce environmental footprints, appealing to a growing segment of environmentally conscious consumers.
| Opportunities | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Integration with digital health platforms and mobile applications | +1.3% | Global | Long Term (2028-2033) |
| Development of highly sensitive and multi-parameter test kits for early and comprehensive insights | +1.1% | North America, Europe | Short to Medium Term (2025-2030) |
| Expansion into emerging economies with growing healthcare awareness and access | +0.9% | Asia Pacific, Latin America, Middle East & Africa | Long Term (2025-2033) |
| Focus on sustainable and eco-friendly product development and packaging | +0.7% | Developed Regions (North America, Europe) | Medium to Long Term (2027-2033) |
| Strategic partnerships with telehealth providers and fertility clinics | +0.6% | Global | Medium Term (2026-2031) |
Pregnancy Rapid Test Kit Market Challenges Impact Analysis
The Pregnancy Rapid Test Kit market faces several critical challenges that demand strategic responses from manufacturers and stakeholders. One significant hurdle is combating the proliferation of counterfeit products, particularly in regions with less stringent regulatory oversight. These unauthorized products not only pose health risks due to unreliable results but also erode consumer trust in legitimate brands and compromise market integrity. Ensuring product authenticity and educating consumers on identifying genuine kits are ongoing efforts crucial for market stability. Additionally, ensuring proper disposal of used test kits presents an environmental challenge. As at-home testing becomes more widespread, the accumulation of plastic and biohazardous waste from these kits requires sustainable disposal solutions or the development of more eco-friendly product designs.
Another challenge involves maintaining accuracy and minimizing user error. While modern kits are designed for ease of use, improper sample collection, incorrect timing, or misinterpretation of faint lines can lead to inaccurate results, causing anxiety or missed opportunities for early care. Public misinformation or lack of comprehensive understanding about test limitations can further complicate this. Moreover, intense market competition and price sensitivity, especially in the over-the-counter segment, can pressure profit margins and hinder investment in advanced research and development. Manufacturers must balance innovation with affordability to remain competitive, navigating the complexities of consumer expectations and healthcare regulations across diverse global markets.
| Challenges | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Combating counterfeit products and ensuring product authenticity | -0.6% | Asia Pacific, Latin America, Middle East & Africa | Continuous |
| Minimizing user error in test administration and result interpretation | -0.5% | Global | Continuous |
| Managing the disposal of medical waste from single-use kits | -0.4% | Developed Regions (North America, Europe) | Long Term (2026-2033) |
| Intense market competition and pricing pressures | -0.3% | Global | Continuous |
| Public misinformation or lack of awareness regarding test limitations | -0.2% | Global | Continuous |
Pregnancy Rapid Test Kit Market - Updated Report Scope
This comprehensive market research report offers an in-depth analysis of the global Pregnancy Rapid Test Kit market, providing a detailed assessment of its current landscape and future growth prospects. The report delves into various aspects including market size estimations, historical trends, and an eight-year forecast period. It meticulously examines key market drivers, restraints, opportunities, and challenges that collectively shape the industry's trajectory. Furthermore, the analysis covers the impact of emerging technologies like Artificial Intelligence (AI) on the market, presenting a forward-looking perspective.
The scope extends to a granular segmentation of the market by various parameters, including product type, technology, distribution channel, and end-user, offering a holistic view of sub-market dynamics. Geographical analysis provides regional insights, highlighting significant growth opportunities and market specifics across North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa. The report also profiles leading market players, offering competitive intelligence and strategic insights vital for stakeholders looking to navigate and capitalize on the evolving Pregnancy Rapid Test Kit market landscape.
| Report Attributes | Report Details |
|---|---|
| Base Year | 2024 |
| Historical Year | 2019 to 2023 |
| Forecast Year | 2025 - 2033 |
| Market Size in 2025 | USD 2.15 Billion |
| Market Forecast in 2033 | USD 3.95 Billion |
| Growth Rate | 7.8% |
| Number of Pages | 257 |
| Key Trends |
|
| Segments Covered |
|
| Key Companies Covered | Global Diagnostics Inc., Precision Health Solutions, Rapid Test Innovations, MediCheck Corp., BioSense Diagnostics, Accurate Health Technologies, OmniTest Systems, SureDetect Medical, LifeCare Diagnostics, BioQuick Solutions, Insight Health Labs, Advanced Diagnostic Kits, VeriTest Biomedical, Prime Medical Devices, Future Health Systems, Diagnostic Innovations Ltd., OmniCare Medical, Precision Bio-Labs, NextGen Diagnostics, RapidVue Health |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Speak to Analyst | Avail customised purchase options to meet your exact research needs. Request For Analyst Or Customization |
Segmentation Analysis
The Pregnancy Rapid Test Kit market is segmented to provide a detailed understanding of its various components and their respective contributions to overall market dynamics. This granular analysis allows for a precise evaluation of consumer preferences, technological adoptions, and distribution strategies across different categories. Understanding these segments is crucial for identifying specific growth pockets, tailoring product development, and optimizing market penetration strategies.
The segmentation primarily breaks down the market by test type, distinguishing between strip, cassette, and midstream formats, each catering to different user preferences regarding ease of use and perceived reliability. Further segmentation by product category differentiates between over-the-counter (OTC) kits, which are widely available for direct consumer purchase, and professional kits, typically used in clinical settings. Distribution channels are also analyzed, encompassing retail pharmacies, online pharmacies, supermarkets, hypermarkets, hospitals, and clinics, reflecting the diverse avenues through which these products reach consumers and healthcare providers. Lastly, the market is segmented by the underlying technology, with Lateral Flow Immunoassay being the dominant method, alongside other immunoassay techniques, highlighting the scientific foundations of these diagnostic tools.
- By Type:
- Strip: Generally the most affordable option, simple to use by dipping into a urine sample.
- Cassette: Offers a more hygienic approach with a dropper to apply urine onto a designated well, providing a window for results.
- Midstream: Designed for direct urine stream application, often considered more convenient and less messy for users.
- By Product:
- Over-the-Counter (OTC): Widely available for direct consumer purchase without a prescription, emphasizing convenience and privacy.
- Professional: Utilized in clinical settings, hospitals, and doctor's offices, often offering higher sensitivity or specific features for healthcare professionals.
- By Distribution Channel:
- Retail Pharmacies: Traditional brick-and-mortar pharmacies serving as a primary point of sale for OTC kits.
- Online Pharmacies: E-commerce platforms offering convenience, competitive pricing, and discreet delivery, experiencing rapid growth.
- Supermarkets & Hypermarkets: Retail chains providing a broad range of products, including rapid test kits, for general consumer accessibility.
- Hospitals & Clinics: Primary channels for professional-grade kits, used for immediate diagnostic purposes in healthcare facilities.
- By Technology:
- Lateral Flow Immunoassay: The most common and widely used technology for rapid pregnancy tests, relying on capillary action to detect hCG.
- Other Immunoassays: Includes other less common or emerging immunoassay techniques used in certain specialized rapid tests.
Regional Highlights
- North America: This region dominates the Pregnancy Rapid Test Kit market, driven by high awareness regarding reproductive health, advanced healthcare infrastructure, and significant disposable incomes. The strong presence of key market players and continuous technological innovation, especially in digital health integration, further solidifies its leading position. Consumers in North America show a strong preference for convenient and reliable at-home testing solutions.
- Europe: Europe represents a mature market characterized by stringent regulatory standards and a strong emphasis on product quality and safety. Increasing adoption of self-testing methods, coupled with rising disposable incomes and robust healthcare spending, contributes to steady market growth. Countries like Germany, the UK, and France are key contributors, driven by a growing focus on early diagnosis and preventative health.
- Asia Pacific (APAC): The Asia Pacific region is projected to exhibit the highest growth rate in the Pregnancy Rapid Test Kit market. This rapid expansion is primarily attributed to its vast population base, improving healthcare access and infrastructure, increasing health awareness campaigns, and a burgeoning middle class with growing disposable income. Countries such as China and India are at the forefront of this growth, offering significant untapped market potential and increasing adoption of convenient diagnostic tools.
- Latin America: This region is experiencing steady growth, supported by improving economic conditions, increasing healthcare expenditure, and rising awareness about women's health. While still developing, the market in Latin America presents opportunities for market players seeking to expand their geographical footprint by offering affordable and accessible rapid test solutions to a growing population.
- Middle East and Africa (MEA): The MEA region is a nascent but emerging market for Pregnancy Rapid Test Kits. Growth is primarily driven by increasing awareness, improving healthcare facilities, and initiatives to enhance maternal health. Challenges such as economic disparities and infrastructure limitations exist, but ongoing investments in healthcare are paving the way for gradual market expansion and adoption of rapid diagnostic tools.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Pregnancy Rapid Test Kit Market.- Global Diagnostics Inc.
- Precision Health Solutions
- Rapid Test Innovations
- MediCheck Corp.
- BioSense Diagnostics
- Accurate Health Technologies
- OmniTest Systems
- SureDetect Medical
- LifeCare Diagnostics
- BioQuick Solutions
- Insight Health Labs
- Advanced Diagnostic Kits
- VeriTest Biomedical
- Prime Medical Devices
- Future Health Systems
- Diagnostic Innovations Ltd.
- OmniCare Medical
- Precision Bio-Labs
- NextGen Diagnostics
- RapidVue Health
Frequently Asked Questions
How accurate are rapid pregnancy tests?
Pregnancy rapid tests are highly accurate, typically reporting over 99% accuracy when used correctly from the day of the missed period. Their accuracy is attributed to their ability to detect the human chorionic gonadotropin (hCG) hormone, which is produced during pregnancy. Factors like testing too early, user error in following instructions, or certain medications can affect results.
What are the latest technologies in pregnancy rapid test kits?
The latest technologies in pregnancy rapid test kits include enhanced sensitivity for earlier detection of hCG, digital integration with smartphone apps for result tracking and cycle analysis, and the development of multi-parameter tests that can also detect ovulation or fertility markers. Some advanced kits also feature AI-powered interpretation for clearer results.
Where can I buy pregnancy rapid test kits?
Pregnancy rapid test kits are widely available over-the-counter at various retail locations. You can purchase them at retail pharmacies, major supermarkets and hypermarkets, and increasingly through online pharmacies for convenience and discreet delivery. Professional-grade kits are typically supplied directly to hospitals and clinics.
What is the future outlook for the pregnancy rapid test kit market?
The future outlook for the pregnancy rapid test kit market is highly positive, projecting significant growth. This growth is driven by continuous technological advancements, increasing demand for convenient home-based diagnostic solutions, growing health awareness globally, and the integration of digital health features. The market is expected to expand with innovations in early detection and personalized health insights.
Are there sustainable options for pregnancy test kits?
Yes, there is a growing trend towards sustainable options for pregnancy test kits. Manufacturers are increasingly exploring eco-friendly materials such as biodegradable plastics and recycled paper for packaging. Some companies are also developing reusable components or offering programs for proper disposal and recycling to minimize environmental impact.