Cell Therapy Market
Cell Therapy Market Size, Scope, Growth, Trends and By Segmentation Types, Applications, Regional Analysis and Industry Forecast (2025-2033)
Report ID : RI_705540 | Last Updated : August 17, 2025 |
Format : ![]()
![]()
![]()
![]()
Cell Therapy Market Size
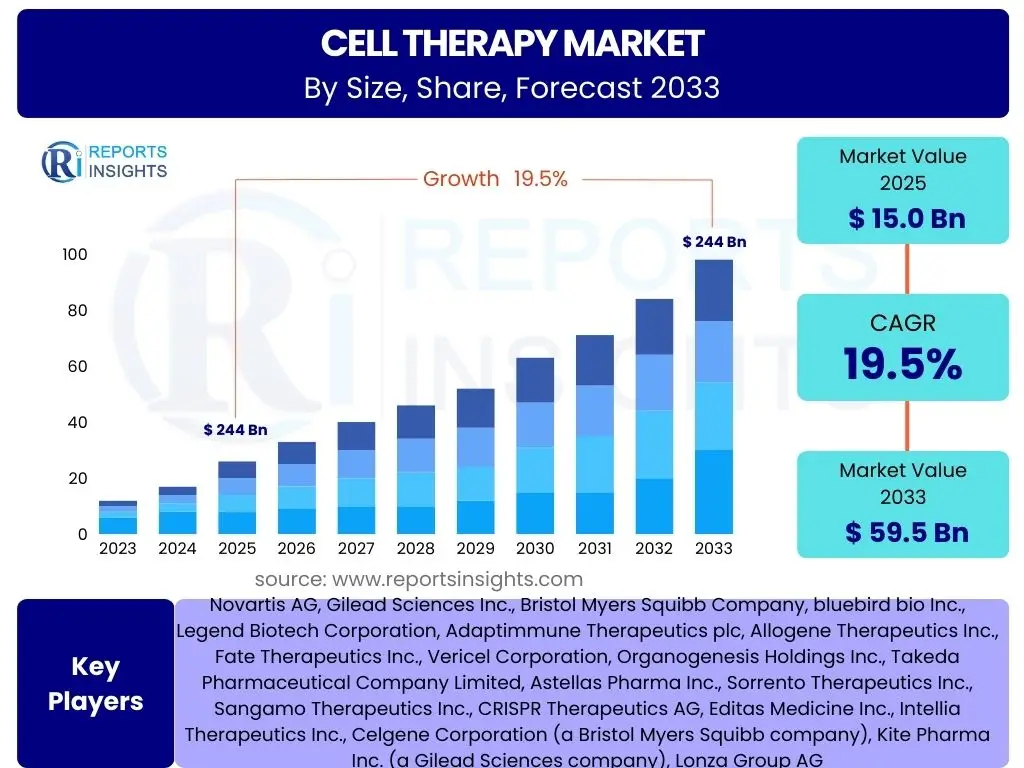
According to Reports Insights Consulting Pvt Ltd, The Cell Therapy Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 19.5% between 2025 and 2033. The market is estimated at USD 15.0 Billion in 2025 and is projected to reach USD 59.5 Billion by the end of the forecast period in 2033.
The significant growth trajectory of the cell therapy market is primarily driven by the increasing prevalence of chronic and life-threatening diseases, coupled with substantial advancements in biotechnology and genetic engineering. These therapies offer potentially curative solutions for conditions previously untreatable or managed only palliatively, marking a paradigm shift in healthcare.
Furthermore, rising investments in research and development by pharmaceutical and biotechnology companies, alongside supportive regulatory frameworks in various countries, are accelerating the commercialization and adoption of novel cell therapies. The expanding pipeline of clinical trials and the approval of new products are also critical factors contributing to this robust market expansion over the forecast period.
Key Cell Therapy Market Trends & Insights
User inquiries about cell therapy trends frequently highlight the shift towards allogeneic approaches, the integration of advanced gene editing technologies, and the growing focus on personalized medicine. There is considerable interest in how manufacturing complexities are being addressed and the influence of regulatory developments on market access. Additionally, users often seek information on the expansion of therapeutic applications beyond oncology, particularly into autoimmune and neurological disorders, reflecting a broader adoption potential for these innovative treatments.
- Shift from autologous to allogeneic cell therapies for enhanced scalability and accessibility.
- Increased integration of CRISPR-Cas9 and other advanced gene editing tools for therapeutic precision.
- Growing emphasis on personalized medicine approaches, tailoring therapies to individual patient profiles.
- Advancements in manufacturing processes, including automation and closed-system bioreactors, to reduce costs and increase efficiency.
- Expansion of therapeutic indications beyond oncology to include autoimmune diseases, neurological disorders, and cardiovascular conditions.
- Rise of combination therapies, leveraging cell therapy with other treatment modalities for improved outcomes.
AI Impact Analysis on Cell Therapy
User questions related to AI's impact on cell therapy frequently revolve around its potential to accelerate drug discovery, optimize clinical trial design, and enhance manufacturing processes. There is keen interest in how artificial intelligence can analyze complex biological data to identify new therapeutic targets, predict patient responses, and personalize treatment strategies. Concerns also emerge regarding data privacy, algorithm transparency, and the validation of AI-driven insights in highly regulated environments like pharmaceutical development.
- Accelerated discovery and identification of novel cell therapy targets and biomarkers through advanced data analytics.
- Optimization of clinical trial design, patient stratification, and predictive modeling for treatment efficacy and safety.
- Enhanced manufacturing process control, quality assurance, and yield optimization through AI-driven automation and predictive maintenance.
- Development of personalized treatment algorithms and dose optimization based on individual patient biological data.
- Improved supply chain management and logistics for highly sensitive and time-critical cell therapy products.
Key Takeaways Cell Therapy Market Size & Forecast
Common user questions regarding key takeaways from the Cell Therapy market size and forecast consistently point to understanding the primary drivers behind its rapid expansion, the most promising therapeutic areas for investment, and the critical challenges that might impede growth. Users are keen to identify segments offering the highest return on investment and assess the long-term sustainability of current growth trends. The impact of regulatory streamlining and technological breakthroughs on future market dynamics also represents a significant area of inquiry.
- The market is poised for substantial growth, driven by increasing disease burden and therapeutic innovation.
- Oncology remains a dominant application area, but significant expansion is expected in autoimmune and neurological disorders.
- Technological advancements in gene editing and allogeneic approaches are critical growth enablers.
- High manufacturing costs and complex regulatory pathways represent significant, yet addressable, challenges.
- Strategic collaborations and partnerships are vital for accelerating research, development, and commercialization.
Cell Therapy Market Drivers Analysis
The cell therapy market is significantly propelled by several key drivers that foster its expansion and adoption. A primary factor is the increasing global prevalence of chronic and life-threatening diseases such as cancers, autoimmune disorders, and degenerative conditions, for which conventional treatments offer limited efficacy or significant side effects. Cell therapies provide a novel, often curative, approach for these unmet medical needs. Concurrently, continuous advancements in gene editing technologies like CRISPR-Cas9 and CAR-T cell engineering are enhancing the precision and therapeutic potential of these treatments, making them more effective and safer. This technological progress is attracting substantial investments from both public and private sectors, fueling robust research and development activities and expanding the clinical pipeline.
Moreover, the supportive regulatory environment in major economies, characterized by expedited review processes and breakthrough therapy designations, is accelerating the approval and market entry of innovative cell therapy products. This regulatory facilitation, coupled with increasing patient awareness and demand for advanced therapeutic options, contributes significantly to market growth. Additionally, the growing number of successful clinical trials and the commercialization of several ground-breaking cell therapy products are building confidence among healthcare providers and patients, further driving market acceptance and penetration globally.
| Drivers | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Increasing Prevalence of Chronic Diseases | +3.0% | Global, particularly North America, Europe, Asia Pacific | Long-term (2025-2033) |
| Advancements in Gene Editing Technologies | +2.5% | Global, especially US, EU, China | Mid to Long-term (2025-2033) |
| Rising R&D Investments and Funding | +2.0% | North America, Europe, select APAC countries | Short to Mid-term (2025-2030) |
| Supportive Regulatory Frameworks | +1.5% | US, EU, Japan, South Korea | Short to Mid-term (2025-2028) |
| Growing Awareness and Patient Acceptance | +1.0% | Global | Mid to Long-term (2028-2033) |
Cell Therapy Market Restraints Analysis
Despite its vast potential, the cell therapy market faces significant restraints that could temper its growth trajectory. One of the most prominent challenges is the exceptionally high cost associated with these therapies, often running into hundreds of thousands of dollars per patient. This high cost is attributable to complex manufacturing processes, rigorous quality control requirements, and extensive research and development expenditures. Such prohibitive pricing poses substantial challenges for healthcare systems, payers, and patients regarding affordability and reimbursement, limiting widespread accessibility and adoption.
Another major restraint is the intricate and demanding manufacturing process for cell therapies. Unlike conventional pharmaceutical products, cell therapies often involve patient-specific (autologous) or donor-specific (allogeneic) biological materials, necessitating highly specialized facilities, skilled personnel, and stringent aseptic conditions. The logistical complexities of collecting, processing, and delivering these living cells within critical timeframes add to the operational burden and cost. Furthermore, ethical concerns, particularly surrounding the use of embryonic stem cells and gene editing technologies, can also create public and regulatory hurdles, although most commercial therapies currently approved utilize adult or induced pluripotent stem cells, or engineered T-cells.
| Restraints | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| High Cost of Cell Therapies | -2.5% | Global, particularly developing regions | Long-term (2025-2033) |
| Complex Manufacturing and Supply Chain | -2.0% | Global | Short to Mid-term (2025-2030) |
| Stringent Regulatory Requirements and Approval Processes | -1.5% | Global, particularly US, EU | Short to Mid-term (2025-2028) |
| Limited Reimbursement Policies | -1.0% | Global, varies by country | Mid to Long-term (2028-2033) |
| Ethical Concerns and Public Perception | -0.5% | Certain religious/conservative regions | Long-term (2025-2033) |
Cell Therapy Market Opportunities Analysis
The cell therapy market presents numerous lucrative opportunities driven by unmet medical needs and technological progress. A significant opportunity lies in the expansion of therapeutic applications beyond oncology, which currently dominates the market. Exploring new indications in autoimmune diseases, neurological disorders, cardiovascular conditions, and regenerative medicine offers vast untapped patient populations and market potential. This diversification is supported by ongoing research identifying new cellular targets and mechanisms of action, broadening the scope of cell therapy interventions.
Furthermore, technological advancements in manufacturing, such as the development of allogeneic (off-the-shelf) therapies and more efficient closed-system bioreactors, are paving the way for reduced production costs and increased scalability. These innovations promise to make cell therapies more accessible and affordable, thereby expanding their market reach. Emerging markets in Asia Pacific and Latin America also present substantial growth opportunities due to their large patient populations, improving healthcare infrastructure, and increasing disposable incomes, attracting investment and clinical trial activities. Strategic collaborations between academic institutions, biotechnology firms, and large pharmaceutical companies are also fostering innovation and accelerating the translation of research into commercial products, creating a robust ecosystem for future growth.
| Opportunities | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Expansion into New Therapeutic Areas (beyond oncology) | +3.5% | Global | Mid to Long-term (2028-2033) |
| Development of Allogeneic Cell Therapies | +3.0% | Global | Mid-term (2025-2030) |
| Technological Advancements in Manufacturing | +2.5% | Global | Short to Mid-term (2025-2030) |
| Emerging Markets Penetration | +2.0% | Asia Pacific, Latin America, MEA | Long-term (2028-2033) |
| Strategic Collaborations and Partnerships | +1.5% | Global | Short to Mid-term (2025-2028) |
Cell Therapy Market Challenges Impact Analysis
The cell therapy market, while promising, grapples with several significant challenges that could impede its rapid expansion. A primary hurdle is the scalability of production, particularly for autologous therapies, which are patient-specific. Manufacturing these therapies often involves highly specialized, labor-intensive, and costly processes that are difficult to scale up to meet growing demand. Ensuring consistent product quality and potency across batches, especially with living biological materials, presents a continuous operational challenge that requires sophisticated quality control measures and substantial investment in infrastructure.
Another key challenge involves the complexities of clinical trial design and execution for cell therapies. These trials often require long follow-up periods to assess durability and long-term safety, which can be expensive and time-consuming. Patient recruitment for specific disease indications can also be challenging, particularly for rare diseases. Additionally, navigating the evolving regulatory landscape across different regions, with varying guidelines for product development, approval, and post-market surveillance, adds another layer of complexity. Finally, the high cost of therapy and the subsequent reimbursement challenges continue to be a barrier to broad market access, necessitating innovative pricing models and robust health economic evidence to demonstrate value to payers.
| Challenges | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Scalability of Manufacturing and Production | -2.5% | Global | Short to Mid-term (2025-2030) |
| Supply Chain and Logistics Complexities | -2.0% | Global | Short to Mid-term (2025-2028) |
| Clinical Trial Design and Long-Term Safety Data | -1.5% | Global | Long-term (2028-2033) |
| Regulatory Harmonization Across Regions | -1.0% | Global | Mid to Long-term (2025-2033) |
| Talent Shortage in Specialized Fields | -0.5% | North America, Europe | Long-term (2025-2033) |
Cell Therapy Market - Updated Report Scope
This market insights report offers a comprehensive analysis of the Cell Therapy Market, encompassing detailed market sizing, growth forecasts, and a deep dive into key trends, drivers, restraints, opportunities, and challenges influencing the industry. The scope extends to a meticulous segmentation analysis based on therapy type, application areas, and end-use sectors, providing granular insights into market dynamics. Furthermore, the report provides a thorough regional outlook and profiles leading companies, offering a holistic view of the competitive landscape and strategic initiatives shaping the future of cell therapy.
| Report Attributes | Report Details |
|---|---|
| Base Year | 2024 |
| Historical Year | 2019 to 2023 |
| Forecast Year | 2025 - 2033 |
| Market Size in 2025 | USD 15.0 Billion |
| Market Forecast in 2033 | USD 59.5 Billion |
| Growth Rate | 19.5% |
| Number of Pages | 247 |
| Key Trends |
|
| Segments Covered |
|
| Key Companies Covered | Novartis AG, Gilead Sciences Inc., Bristol Myers Squibb Company, bluebird bio Inc., Legend Biotech Corporation, Adaptimmune Therapeutics plc, Allogene Therapeutics Inc., Fate Therapeutics Inc., Vericel Corporation, Organogenesis Holdings Inc., Takeda Pharmaceutical Company Limited, Astellas Pharma Inc., Sorrento Therapeutics Inc., Sangamo Therapeutics Inc., CRISPR Therapeutics AG, Editas Medicine Inc., Intellia Therapeutics Inc., Celgene Corporation (a Bristol Myers Squibb company), Kite Pharma Inc. (a Gilead Sciences company), Lonza Group AG |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Speak to Analyst | Avail customised purchase options to meet your exact research needs. Request For Analyst Or Customization |
Segmentation Analysis
The Cell Therapy Market is comprehensively segmented to provide a detailed understanding of its diverse components and underlying market dynamics. This segmentation facilitates a granular analysis of various market aspects, allowing stakeholders to identify key growth areas, emerging opportunities, and competitive landscapes within specific categories. The primary segmentation is conducted by therapy type, application, and end-use, reflecting the varied nature of cell therapy products and their utilization across different healthcare settings and disease areas.
Understanding these segments is crucial for strategic planning, product development, and market entry decisions. Each segment exhibits unique growth drivers and challenges, influenced by regulatory frameworks, technological advancements, and patient demographics. For instance, the distinction between autologous and allogeneic therapies highlights differing manufacturing complexities and scalability potentials, while application-based segmentation reveals the most lucrative therapeutic areas and unmet medical needs driving adoption.
- By Type:
- Autologous: Therapies derived from a patient's own cells.
- Allogeneic: Therapies derived from donor cells, offering off-the-shelf potential.
- By Application:
- Oncology: Including treatments for various cancers like Leukemia, Lymphoma, Myeloma, and Solid Tumors.
- Autoimmune Diseases: Such as Rheumatoid Arthritis and Lupus.
- Musculoskeletal Disorders: Covering Osteoarthritis and Cartilage Repair.
- Cardiovascular Diseases: Targeting heart-related conditions.
- Neurological Disorders: Addressing ailments like Parkinson's, Alzheimer's, and Spinal Cord Injury.
- Others: Including applications in Dermatology and Gastrointestinal disorders.
- By End-Use:
- Hospitals: Primary settings for acute care and specialized treatments.
- Ambulatory Surgical Centers (ASCs): Facilities offering outpatient surgical procedures.
- Specialty Clinics: Focused on specific diseases or therapeutic areas.
- Research & Academic Institutes: Driving fundamental research and early-stage clinical trials.
- Biopharmaceutical Companies: Developing and commercializing cell therapy products.
- Contract Research Organizations (CROs): Providing specialized support for clinical trials and development.
Regional Highlights
- North America: Expected to dominate the cell therapy market due to significant investments in research and development, a high prevalence of chronic diseases, a well-established healthcare infrastructure, and the presence of major pharmaceutical and biotechnology companies. Favorable reimbursement policies and a robust pipeline of clinical trials further contribute to its leading position.
- Europe: Represents a substantial market share, driven by increasing government funding for cell and gene therapy research, a strong academic research base, and a growing number of approved cell therapy products. Key countries like Germany, the UK, and France are at the forefront of adopting advanced therapies and fostering innovation.
- Asia Pacific (APAC): Projected to exhibit the highest growth rate during the forecast period, fueled by a large and aging population, rising healthcare expenditure, improving healthcare infrastructure, and increasing awareness of advanced therapies. Countries like China, Japan, and South Korea are emerging as key players, with growing R&D activities and supportive government initiatives.
- Latin America: Expected to experience steady growth, primarily due to increasing healthcare access, a rising burden of chronic diseases, and a developing interest in advanced therapeutic options. However, market expansion might be tempered by economic volatility and varying regulatory frameworks.
- Middle East and Africa (MEA): Showing nascent growth, driven by increasing healthcare investments and efforts to diversify economies, especially in the GCC countries. Challenges include limited research infrastructure and a slower adoption rate compared to more developed regions, though opportunities exist for partnerships and technology transfer.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Cell Therapy Market.- Novartis AG
- Gilead Sciences Inc.
- Bristol Myers Squibb Company
- bluebird bio Inc.
- Legend Biotech Corporation
- Adaptimmune Therapeutics plc
- Allogene Therapeutics Inc.
- Fate Therapeutics Inc.
- Vericel Corporation
- Organogenesis Holdings Inc.
- Takeda Pharmaceutical Company Limited
- Astellas Pharma Inc.
- Sorrento Therapeutics Inc.
- Sangamo Therapeutics Inc.
- CRISPR Therapeutics AG
- Editas Medicine Inc.
- Intellia Therapeutics Inc.
- Celgene Corporation (a Bristol Myers Squibb company)
- Kite Pharma Inc. (a Gilead Sciences company)
- Lonza Group AG
Frequently Asked Questions
Analyze common user questions about the Cell Therapy market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is cell therapy?
Cell therapy is a medical treatment that involves injecting cellular material into a patient to treat or prevent disease. This can include intact living cells, such as T-cells, stem cells, or other cell types, which may be modified or engineered to perform specific functions, like targeting cancer cells or repairing damaged tissues.
What are the primary applications of cell therapy?
The primary applications of cell therapy currently include oncology, particularly for blood cancers like leukemia and lymphoma using CAR-T cell therapies. Expanding applications include autoimmune diseases, neurological disorders, cardiovascular conditions, and various regenerative medicine indications like cartilage repair and wound healing.
What are the main challenges facing the cell therapy market?
Key challenges include the high cost of development and treatment, complex manufacturing processes that hinder scalability, stringent regulatory requirements, logistical complexities in supply chain management for living cells, and the need for long-term safety and efficacy data from clinical trials.
How is artificial intelligence impacting cell therapy development?
AI is transforming cell therapy by accelerating target discovery, optimizing clinical trial design and patient selection, enhancing manufacturing efficiency through automation and predictive analytics, and enabling more personalized treatment strategies by analyzing complex biological data to predict responses and outcomes.
What is the difference between autologous and allogeneic cell therapies?
Autologous cell therapy uses a patient's own cells, which are collected, modified, and then reinfused, ensuring no immune rejection but presenting manufacturing and logistical challenges. Allogeneic cell therapy uses cells from a healthy donor, offering "off-the-shelf" availability and scalability, though it requires strategies to mitigate immune rejection.

