CAR T Cell Therapy Market
CAR T Cell Therapy Market Size, Scope, Growth, Trends and By Segmentation Types, Applications, Regional Analysis and Industry Forecast (2025-2033)
Report ID : RI_705670 | Last Updated : August 17, 2025 |
Format : ![]()
![]()
![]()
![]()
CAR T Cell Therapy Market Size
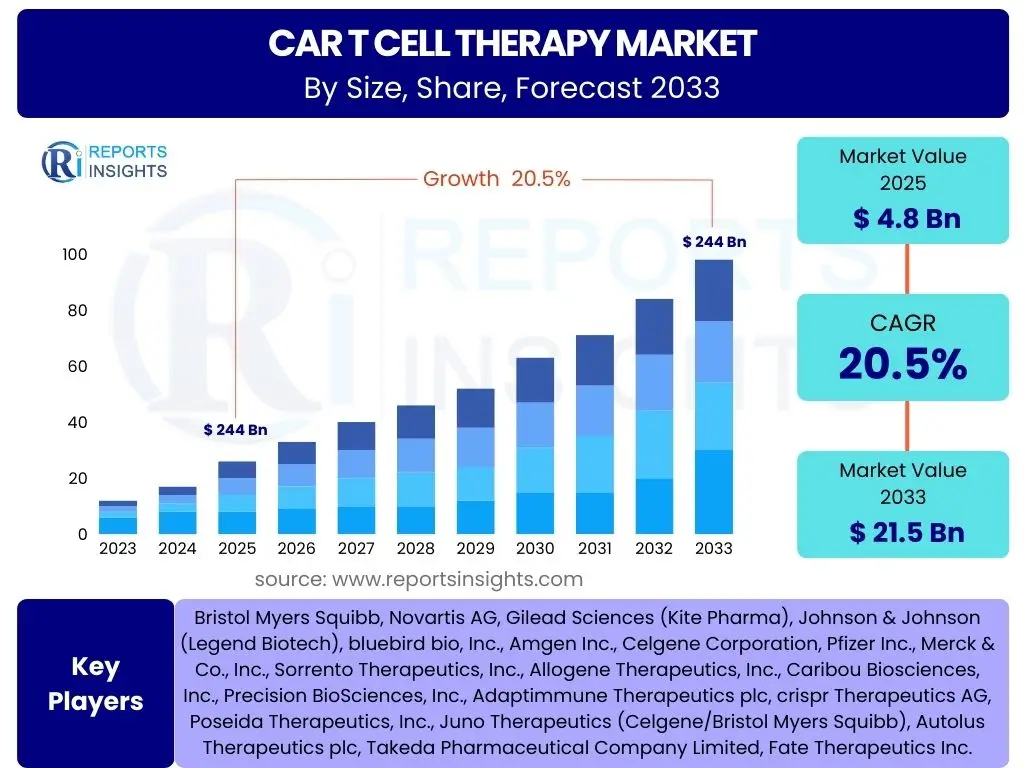
According to Reports Insights Consulting Pvt Ltd, The CAR T Cell Therapy Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 20.5% between 2025 and 2033. The market is estimated at USD 4.8 Billion in 2025 and is projected to reach USD 21.5 Billion by the end of the forecast period in 2033. This robust growth trajectory is primarily driven by the expanding applications of CAR T cell therapies beyond hematological malignancies, coupled with continuous advancements in gene editing technologies and manufacturing processes. The increasing prevalence of various types of cancers globally, especially those unresponsive to conventional treatments, further underpins the significant market expansion.
The substantial increase in market valuation reflects a growing investment in research and development by pharmaceutical and biotechnology companies, alongside rising patient access to these innovative therapies. Despite the high cost associated with CAR T cell treatments, their curative potential in specific patient populations is a key factor contributing to their adoption and market growth. Strategic collaborations and partnerships aimed at accelerating therapy development and improving scalability are also playing a crucial role in shaping the market's future landscape, paving the way for broader therapeutic applications and improved patient outcomes.
Key CAR T Cell Therapy Market Trends & Insights
Users frequently inquire about the evolving landscape of CAR T cell therapy, seeking to understand the most impactful trends that are shaping its development and commercialization. Common questions revolve around the expansion of therapeutic targets, advancements in manufacturing, and strategies to mitigate treatment challenges. The market is witnessing a significant shift towards broadening the scope of CAR T cell applications beyond approved hematological cancers, with considerable research efforts directed towards solid tumors and autoimmune diseases. This diversification is critical for unlocking new market opportunities and addressing a wider range of unmet medical needs, providing innovative treatment options where traditional therapies have failed.
Another prominent trend is the strong focus on developing allogeneic, or "off-the-shelf," CAR T cell therapies. These therapies aim to overcome the complexities, high costs, and lengthy manufacturing times associated with autologous treatments, which are derived from a patient's own cells. Allogeneic approaches promise greater accessibility, reduced production costs, and immediate availability, potentially revolutionizing the scalability and affordability of CAR T cell therapy. Additionally, technological advancements in gene editing, such as CRISPR/Cas9, are enabling more precise and efficient engineering of T cells, leading to improved efficacy and safety profiles, thereby enhancing the overall therapeutic potential and reducing the incidence of severe adverse events.
- Expansion into solid tumor indications to address significant unmet medical needs.
- Development of allogeneic "off-the-shelf" CAR T cell products for increased accessibility and reduced manufacturing time.
- Integration of advanced gene editing technologies (e.g., CRISPR) for enhanced precision and safety.
- Focus on improving CAR T cell persistence and reducing neurotoxicity and cytokine release syndrome (CRS).
- Emergence of next-generation CAR T constructs with novel co-stimulatory domains and suicide genes.
- Increasing strategic collaborations and partnerships between academic institutions, biotech firms, and pharmaceutical giants.
- Digitalization and automation in CAR T cell manufacturing to improve efficiency and reduce costs.
AI Impact Analysis on CAR T Cell Therapy
Common user questions regarding AI's impact on CAR T Cell Therapy center on its potential to accelerate discovery, optimize development processes, and enhance patient outcomes. Users are keen to understand how artificial intelligence and machine learning algorithms are being leveraged to address the inherent complexities of CAR T cell design, manufacturing, and clinical application. AI's ability to analyze vast biological datasets, identify novel therapeutic targets, and predict patient responses is recognized as a transformative force, enabling more rational design of CAR constructs and more effective stratification of patients for therapy. This capability significantly reduces the time and resources traditionally required for target validation and preclinical screening.
Furthermore, AI is playing a pivotal role in streamlining the intricate manufacturing processes of CAR T cell therapies, from cell isolation and expansion to quality control and logistics. Machine learning algorithms can monitor and optimize critical parameters during cell production, ensuring consistent quality, scalability, and cost-effectiveness. In the clinical setting, AI-driven analytics assist in real-time monitoring of patients for adverse events, predicting treatment efficacy, and personalizing dosing regimens, thereby enhancing safety and maximizing therapeutic benefit. The integration of AI tools promises to unlock new efficiencies across the entire CAR T cell therapy pipeline, from initial discovery to commercial deployment and patient management, making these advanced treatments more accessible and effective.
- Accelerated discovery of novel CAR T cell targets through bioinformatics and machine learning analysis of omics data.
- Optimized CAR T cell construct design by predicting efficacy, specificity, and off-target effects.
- Enhanced manufacturing processes through AI-driven automation, process control, and quality assurance.
- Improved patient selection and stratification using predictive analytics based on clinical and genetic data.
- Real-time monitoring and management of adverse events like Cytokine Release Syndrome (CRS) and neurotoxicity.
- Development of personalized treatment strategies and adaptive dosing regimens.
- Identification of biomarkers for treatment response and resistance, guiding future therapy development.
Key Takeaways CAR T Cell Therapy Market Size & Forecast
Users frequently inquire about the overall outlook and significant conclusions drawn from the CAR T Cell Therapy market size and forecast, seeking concise summaries of market potential, growth drivers, and critical success factors. A primary takeaway is the exceptionally high growth trajectory predicted for the market, indicating strong confidence in CAR T cell therapies as a transformative treatment modality, particularly for patients with refractory cancers. This aggressive growth is underpinned by the significant clinical benefits observed in approved indications and the expanding pipeline for new applications, signaling a robust and dynamic future for the sector. The market's expansion is also a testament to continuous innovation in cellular engineering and the growing investment landscape.
Another crucial insight is the sustained research and development focus on overcoming current limitations, such as high treatment costs, complex manufacturing, and the challenge of treating solid tumors. Success in these areas will be pivotal for unlocking the full market potential and ensuring broader patient accessibility. The drive towards allogeneic platforms and advanced manufacturing techniques suggests a future where CAR T cell therapies are more scalable and affordable. Furthermore, the increasing global prevalence of cancer and the growing demand for highly effective, personalized treatment options continue to serve as fundamental demand drivers, ensuring sustained investment and innovation in this rapidly evolving therapeutic area.
- The CAR T Cell Therapy market is set for rapid expansion, driven by clinical successes and a robust R&D pipeline.
- Significant growth is anticipated from the expansion into solid tumor indications and autoimmune diseases.
- Cost reduction and scalability of manufacturing remain critical factors influencing market penetration and accessibility.
- Allogeneic CAR T cell therapies represent a major future growth opportunity due to their "off-the-shelf" potential.
- Regulatory support and increasing healthcare expenditure in key regions will continue to fuel market growth.
- Patient access and reimbursement policies are crucial for widespread adoption and market maturation.
CAR T Cell Therapy Market Drivers Analysis
The CAR T cell therapy market is significantly propelled by a confluence of critical drivers, primarily the escalating global incidence of cancer, particularly hematological malignancies where these therapies have shown remarkable success. The urgent unmet medical need for effective treatments in patients with relapsed or refractory cancers, who have exhausted conventional options, drives demand for these innovative therapies. Furthermore, continuous advancements in biotechnology and genetic engineering techniques enable the development of more sophisticated and safer CAR T cell constructs, broadening their therapeutic window and improving efficacy. These technological leaps are crucial for expanding the applicability of CAR T cells beyond their current scope, fostering innovation that addresses previously intractable diseases.
Favorable regulatory frameworks and increasing investments in research and development by pharmaceutical companies, biotechnology firms, and academic institutions worldwide also contribute substantially to market acceleration. Governments and regulatory bodies are streamlining approval processes for advanced cell and gene therapies, recognizing their potential to revolutionize patient care. Moreover, rising awareness among healthcare professionals and patients about the transformative potential of CAR T cell therapies, coupled with improving reimbursement landscapes in developed economies, encourages wider adoption and market penetration. These factors collectively create a conducive environment for sustained growth, driving both the supply and demand sides of the CAR T cell therapy market.
| Drivers | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Rising Incidence of Cancer & Relapsed/Refractory Cases | +2.8% | Global | Long-term (2025-2033) |
| Technological Advancements in Gene Editing & Cell Engineering | +2.5% | North America, Europe, Asia Pacific | Mid to Long-term (2025-2033) |
| Increasing R&D Investments & Strategic Collaborations | +2.0% | Global | Long-term (2025-2033) |
| Favorable Regulatory Policies & Accelerated Approvals | +1.8% | North America, Europe | Mid-term (2025-2029) |
| Growing Awareness & Improving Reimbursement Policies | +1.5% | Developed Economies | Mid to Long-term (2025-2033) |
CAR T Cell Therapy Market Restraints Analysis
Despite the immense promise of CAR T cell therapy, several significant restraints impede its broader market penetration and adoption. The most prominent challenge is the exceptionally high cost associated with these treatments, often reaching hundreds of thousands of dollars per patient. This exorbitant price point poses a substantial burden on healthcare systems and limits patient access, particularly in regions with less developed healthcare infrastructure or limited reimbursement policies. The complex and highly personalized manufacturing process for autologous CAR T cells, involving patient-specific cell collection, genetic modification, and reinfusion, contributes significantly to these high costs and logistical complexities.
Furthermore, the risk of severe adverse events, such as Cytokine Release Syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), remains a critical concern. While these side effects are generally manageable with expert medical care, they necessitate specialized treatment centers and highly trained healthcare professionals, further increasing the cost and complexity of patient management. Limited scalability of manufacturing, the lengthy vein-to-vein time for autologous products, and the challenges in achieving sustained efficacy in solid tumors also represent significant hurdles. Overcoming these restraints through innovation in manufacturing, cost-reduction strategies, and improved safety profiles is crucial for the long-term sustainability and widespread accessibility of CAR T cell therapies.
| Restraints | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| High Treatment Costs & Limited Reimbursement | -2.2% | Global | Long-term (2025-2033) |
| Complex Manufacturing & Logistical Challenges | -1.8% | Global | Mid-term (2025-2029) |
| Risk of Severe Adverse Events (CRS, ICANS) | -1.5% | Global | Long-term (2025-2033) |
| Limited Efficacy in Solid Tumors | -1.3% | Global | Long-term (2025-2033) |
| Patient Accessibility & Treatment Center Limitations | -1.0% | Emerging Economies | Mid to Long-term (2025-2033) |
CAR T Cell Therapy Market Opportunities Analysis
The CAR T cell therapy market is ripe with numerous opportunities poised to drive significant expansion and innovation. A primary opportunity lies in the vigorous expansion of therapeutic applications beyond current indications, specifically into solid tumors and a range of autoimmune diseases. While treating solid tumors remains a complex challenge, ongoing research into novel target antigens, improved CAR designs, and combination therapies holds immense promise for unlocking a much larger patient population. Similarly, initial successes in applying CAR T technology to autoimmune conditions like lupus suggest a broad, untapped market, offering a completely new paradigm for managing chronic debilitating diseases where conventional immunosuppression often falls short.
The development of allogeneic, "off-the-shelf" CAR T cell therapies represents another transformative opportunity. These standardized products can be manufactured in advance, stored, and administered quickly to patients, overcoming the logistical hurdles, high costs, and lengthy production times associated with autologous treatments. This shift promises to significantly improve patient access, reduce costs, and enhance scalability, democratizing CAR T cell therapy. Furthermore, the exploration of combination therapies, integrating CAR T cells with checkpoint inhibitors, chemotherapy, or other immunotherapies, offers avenues for enhanced efficacy and broader applicability. Strategic investments in manufacturing automation, digitalization, and localized production facilities are also key opportunities for improving efficiency and reducing the overall cost of goods, further driving market growth and accessibility.
| Opportunities | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Expansion into Solid Tumor Indications | +3.0% | Global | Long-term (2028-2033) |
| Development of Allogeneic (Off-the-Shelf) CAR T Cells | +2.7% | Global | Mid to Long-term (2026-2033) |
| Application in Autoimmune Diseases | +2.0% | North America, Europe | Long-term (2029-2033) |
| Combination Therapies to Enhance Efficacy | +1.8% | Global | Mid to Long-term (2027-2033) |
| Automation & Cost Reduction in Manufacturing | +1.5% | Global | Mid-term (2025-2029) |
CAR T Cell Therapy Market Challenges Impact Analysis
The CAR T cell therapy market faces several intricate challenges that demand innovative solutions for sustained growth and broader adoption. One significant hurdle is the inherent complexity and logistical intensity of the manufacturing process, particularly for autologous therapies. This involves apheresis, shipping, cell processing in specialized facilities, and reinfusion, each step demanding rigorous quality control and precise coordination. The "vein-to-vein" time, which can extend over several weeks, poses a critical challenge for patients with rapidly progressing diseases and adds substantial operational costs. Ensuring consistent product quality and sterility across global manufacturing sites further compounds these complexities, impacting scalability and affordability.
Another major challenge is the management of severe adverse events, primarily cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). While effective management strategies have improved patient safety, these toxicities necessitate specialized hospital settings and highly trained medical personnel, limiting the number of treatment centers and thus patient access. Furthermore, the high cost of therapy and the evolving reimbursement landscape present significant barriers, especially in healthcare systems that are still adapting to novel gene therapies. Achieving sustained clinical efficacy in certain patient populations and overcoming mechanisms of resistance, such as antigen escape, also remain critical scientific and clinical challenges that require continuous research and development efforts to broaden the therapeutic reach of CAR T cell therapies.
| Challenges | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Manufacturing Scalability & High Vein-to-Vein Time | -2.0% | Global | Mid-term (2025-2029) |
| Management of Acute Toxicities & Adverse Events | -1.7% | Global | Long-term (2025-2033) |
| Accessibility & Limited Number of Treatment Centers | -1.5% | Emerging & Developed Economies | Mid to Long-term (2025-2033) |
| High Cost of Therapy & Reimbursement Challenges | -1.2% | Global | Long-term (2025-2033) |
| Antigen Escape & Resistance Mechanisms | -1.0% | Global | Long-term (2027-2033) |
CAR T Cell Therapy Market - Updated Report Scope
This comprehensive market research report provides an in-depth analysis of the CAR T Cell Therapy Market, offering valuable insights into its current status, growth drivers, restraints, opportunities, and future outlook. The scope of the report encompasses a detailed examination of market size and forecast, key market trends, the impact of artificial intelligence, and a granular segmentation analysis across various parameters. It aims to equip stakeholders with a thorough understanding of the market dynamics, competitive landscape, and strategic implications for investment and development within the rapidly evolving field of cell and gene therapy.
| Report Attributes | Report Details |
|---|---|
| Base Year | 2024 |
| Historical Year | 2019 to 2023 |
| Forecast Year | 2025 - 2033 |
| Market Size in 2025 | USD 4.8 Billion |
| Market Forecast in 2033 | USD 21.5 Billion |
| Growth Rate | 20.5% |
| Number of Pages | 245 |
| Key Trends |
|
| Segments Covered |
|
| Key Companies Covered | Bristol Myers Squibb, Novartis AG, Gilead Sciences (Kite Pharma), Johnson & Johnson (Legend Biotech), bluebird bio, Inc., Amgen Inc., Celgene Corporation, Pfizer Inc., Merck & Co., Inc., Sorrento Therapeutics, Inc., Allogene Therapeutics, Inc., Caribou Biosciences, Inc., Precision BioSciences, Inc., Adaptimmune Therapeutics plc, crispr Therapeutics AG, Poseida Therapeutics, Inc., Juno Therapeutics (Celgene/Bristol Myers Squibb), Autolus Therapeutics plc, Takeda Pharmaceutical Company Limited, Fate Therapeutics Inc. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Speak to Analyst | Avail customised purchase options to meet your exact research needs. Request For Analyst Or Customization |
Segmentation Analysis
The CAR T cell therapy market is segmented to provide a granular understanding of its diverse components and drivers. This segmentation allows for a detailed analysis of market dynamics based on target antigens, specific indications, therapeutic areas, type of cell therapy, and end-use applications. By breaking down the market into these distinct categories, stakeholders can identify niche opportunities, understand specific demand patterns, and tailor their strategies to address the unique needs of different patient populations and healthcare settings. The current market is predominantly driven by therapies targeting CD19 and BCMA antigens, which have demonstrated significant success in hematological malignancies.
However, future growth is anticipated from the expansion into novel target antigens and a broader range of indications, particularly solid tumors and autoimmune diseases, which represent vast untapped markets. The distinction between autologous and allogeneic therapy types is crucial, with allogeneic approaches poised to revolutionize accessibility and cost-effectiveness. Furthermore, the analysis of end-use segments, encompassing hospitals, cancer centers, and research institutes, highlights the diverse ecosystem involved in the delivery and development of these advanced therapies, pointing to opportunities for specialized service providers and contract manufacturing organizations.
- By Target Antigen:
- CD19
- BCMA
- CD22
- Others (e.g., CD30, GPC3)
- By Indication:
- Leukemia
- Acute Lymphoblastic Leukemia (ALL)
- Chronic Lymphocytic Leukemia (CLL)
- Lymphoma
- Non-Hodgkin Lymphoma (NHL)
- Hodgkin Lymphoma
- Multiple Myeloma
- Solid Tumors (e.g., Glioblastoma, Ovarian Cancer, Lung Cancer)
- Autoimmune Diseases (e.g., Systemic Lupus Erythematosus)
- Leukemia
- By Therapeutic Area:
- Oncology
- Autoimmune Diseases
- By Type:
- Autologous CAR T Cell Therapy
- Allogeneic CAR T Cell Therapy
- By End-Use:
- Hospitals
- Cancer Centers
- Research Institutes
- Contract Manufacturing Organizations (CMOs)
Regional Highlights
- North America: This region dominates the CAR T Cell Therapy market, primarily driven by significant investments in research and development, a robust healthcare infrastructure, and the presence of leading pharmaceutical and biotechnology companies. Favorable reimbursement policies, a high prevalence of cancer, and increasing adoption of advanced therapies further solidify its leading position. The United States, in particular, is at the forefront of clinical trials and commercialization of CAR T cell products.
- Europe: Europe represents a substantial market for CAR T Cell Therapy, characterized by a growing number of approved therapies, increasing awareness among medical professionals, and expanding patient access. Countries like Germany, France, and the UK are witnessing strong growth due to government initiatives supporting cell and gene therapy research and a well-developed network of cancer treatment centers. Regulatory harmonization across the European Union is also facilitating market expansion.
- Asia Pacific (APAC): The APAC region is projected to experience the fastest growth in the CAR T Cell Therapy market, fueled by increasing healthcare expenditure, a large and aging population, and a rising prevalence of cancer. Emerging economies like China, Japan, and South Korea are rapidly investing in advanced healthcare technologies and developing their own CAR T cell therapy pipelines. Strategic collaborations and efforts to reduce treatment costs are key drivers in this region.
- Latin America: This region is an emerging market for CAR T Cell Therapy, with increasing awareness and nascent adoption. Challenges related to high treatment costs and limited healthcare infrastructure are being gradually addressed through public-private partnerships and local initiatives to improve access to advanced therapies. Brazil and Mexico are showing early signs of growth and investment in cell therapy research.
- Middle East and Africa (MEA): The MEA region is currently a relatively small but growing market for CAR T Cell Therapy. Growth is primarily driven by increasing healthcare spending in Gulf Cooperation Council (GCC) countries and a rising demand for advanced cancer treatments. However, limited access to specialized treatment centers and the high cost of therapy remain significant barriers that are slowly being overcome through strategic healthcare reforms and international collaborations.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the CAR T Cell Therapy Market.- Bristol Myers Squibb
- Novartis AG
- Gilead Sciences (Kite Pharma)
- Johnson & Johnson (Legend Biotech)
- bluebird bio, Inc.
- Amgen Inc.
- Celgene Corporation
- Pfizer Inc.
- Merck & Co., Inc.
- Sorrento Therapeutics, Inc.
- Allogene Therapeutics, Inc.
- Caribou Biosciences, Inc.
- Precision BioSciences, Inc.
- Adaptimmune Therapeutics plc
- crispr Therapeutics AG
- Poseida Therapeutics, Inc.
- Juno Therapeutics (Celgene/Bristol Myers Squibb)
- Autolus Therapeutics plc
- Takeda Pharmaceutical Company Limited
- Fate Therapeutics Inc.
Frequently Asked Questions
Analyze common user questions about the CAR T Cell Therapy market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is CAR T Cell Therapy?
CAR T Cell Therapy is a revolutionary form of immunotherapy that involves engineering a patient's own T cells (a type of immune cell) to recognize and attack cancer cells. These T cells are genetically modified in a laboratory to express a chimeric antigen receptor (CAR) on their surface, enabling them to specifically target antigens found on tumor cells. Once reinfused into the patient, these "living drugs" proliferate and launch a highly targeted immune response against the cancer, offering a personalized and potent treatment option for certain types of advanced cancers.
How does CAR T Cell Therapy work?
The process of CAR T Cell Therapy typically begins with a patient's T cells being collected through a process called apheresis. These cells are then sent to a specialized manufacturing facility where they are genetically modified using a viral vector to produce CARs on their surface. These CARs enable the T cells to specifically bind to antigens present on cancer cells. After expansion to large numbers, the modified CAR T cells are infused back into the patient, where they actively seek out and destroy cancer cells, leading to sustained remission in many cases. The therapy harnesses the body's own immune system to fight the disease.
What types of cancer are treated with CAR T Cell Therapy?
Currently, the primary indications for approved CAR T Cell Therapy include specific blood cancers, such as certain types of B-cell acute lymphoblastic leukemia (ALL) in children and young adults, and various forms of lymphoma, including diffuse large B-cell lymphoma (DLBCL) and mantle cell lymphoma (MCL) in adults. It is also approved for multiple myeloma. Research is actively exploring its potential in treating other hematological malignancies and, more notably, a wide range of solid tumors and autoimmune diseases. Expansion into these new indications is a significant focus of ongoing clinical trials.
What are the potential side effects of CAR T Cell Therapy?
While highly effective, CAR T Cell Therapy can cause severe side effects due to its potent immune activation. The most common and significant adverse events include Cytokine Release Syndrome (CRS) and Immune effector Cell-Associated Neurotoxicity Syndrome (ICANS). CRS is a systemic inflammatory response that can lead to fever, low blood pressure, difficulty breathing, and organ dysfunction. ICANS involves neurological symptoms ranging from confusion and headaches to seizures and speech difficulties. These side effects usually require careful monitoring and specialized medical management, often in an intensive care setting, but are generally reversible with timely intervention.
What is the future outlook for the CAR T Cell Therapy market?
The future outlook for the CAR T Cell Therapy market is exceptionally promising, with projections indicating robust growth and significant innovation. Key trends include the development of allogeneic "off-the-shelf" CAR T cell products, which promise to improve accessibility and reduce manufacturing complexities and costs. There is also a strong focus on expanding the therapy's application to solid tumors and autoimmune diseases, which represent vast untapped markets. Continuous advancements in gene editing technologies, automation in manufacturing, and increasing global healthcare investments are expected to drive further market expansion, making CAR T Cell Therapy a cornerstone of advanced personalized medicine.

