HIV Diagnostic Market
HIV Diagnostic Market Size, Scope, Growth, Trends and By Segmentation Types, Applications, Regional Analysis and Industry Forecast (2025-2033)
Report ID : RI_700779 | Last Updated : July 28, 2025 |
Format : ![]()
![]()
![]()
![]()
HIV Diagnostic Market Size
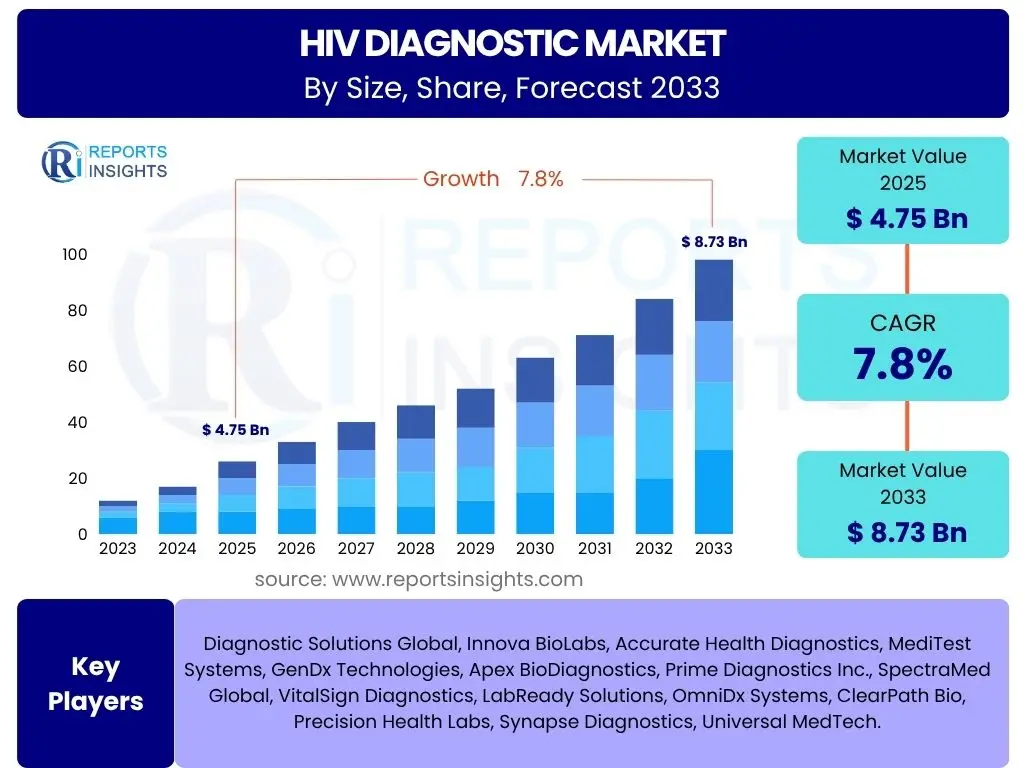
According to Reports Insights Consulting Pvt Ltd, The HIV Diagnostic Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 7.8% between 2025 and 2033. The market is estimated at USD 4.75 billion in 2025 and is projected to reach USD 8.73 billion by the end of the forecast period in 2033. This growth is driven by increasing global awareness programs, advancements in diagnostic technologies, and the rising prevalence of HIV, particularly in developing regions. The market demonstrates a robust expansion trajectory, reflecting concerted efforts in disease surveillance and management worldwide.
The expansion of the HIV diagnostic market is further supported by the growing demand for early detection and efficient monitoring solutions. As healthcare systems globally emphasize preventative care and rapid intervention, the adoption of advanced diagnostic tools such as point-of-care tests and highly sensitive nucleic acid tests (NAT) is accelerating. This technological progression, coupled with strategic public health initiatives aimed at expanding access to testing, underpins the positive outlook for market valuation and volume growth throughout the forecast period.
Key HIV Diagnostic Market Trends & Insights
Common user inquiries regarding HIV diagnostic market trends often center on the emergence of novel testing methodologies, the shift towards decentralized testing, and the integration of digital health solutions. Users seek to understand how these trends are improving accuracy, accessibility, and patient outcomes, as well as their implications for market dynamics. Key insights reveal a significant push towards innovative, user-friendly diagnostic platforms that cater to diverse healthcare settings and global health objectives.
- Increased adoption of rapid diagnostic tests (RDTs) for widespread screening, particularly in resource-limited settings.
- Growth in demand for point-of-care (POC) testing, enabling quicker diagnosis and linkage to care outside traditional laboratory environments.
- Advancements in nucleic acid testing (NAT) for early and highly accurate detection of HIV infection, including in acute phases.
- Integration of digital health platforms and telemedicine for remote consultation, result delivery, and patient follow-up.
- Development of multi-analyte tests that can detect HIV alongside other sexually transmitted infections (STIs).
- Focus on cost-effective diagnostic solutions to enhance affordability and accessibility in low and middle-income countries.
AI Impact Analysis on HIV Diagnostic
Users frequently inquire about the transformative potential of artificial intelligence (AI) in HIV diagnostics, focusing on its ability to enhance testing accuracy, expedite result analysis, and support epidemiological tracking. There is significant interest in how AI algorithms can interpret complex diagnostic data, predict disease progression, and even contribute to the development of new diagnostic markers. Concerns often revolve around data privacy, ethical implications of AI deployment in healthcare, and the need for robust validation protocols to ensure reliability and minimize biases.
AI's influence extends beyond mere data processing, promising to revolutionize diagnostic workflows. It can facilitate the automation of laboratory processes, reduce human error, and enable real-time data analysis for large populations, thereby improving public health responses. The application of machine learning in interpreting imaging data or complex genetic sequences for HIV detection and monitoring is a burgeoning area. Furthermore, AI-powered predictive analytics could assist in identifying high-risk populations and guiding targeted intervention strategies, optimizing resource allocation in global HIV prevention and treatment efforts.
- Enhanced accuracy and sensitivity of diagnostic tests through AI-powered image analysis and data interpretation.
- Accelerated processing of test results and reduction in turnaround times by automating laboratory workflows.
- Predictive analytics for identifying high-risk individuals and forecasting disease outbreaks, enabling proactive public health interventions.
- Development of novel diagnostic algorithms capable of detecting subtle markers or patterns missed by traditional methods.
- Improved efficiency in resource-limited settings by optimizing testing strategies and supply chain management through AI-driven insights.
- Facilitation of personalized treatment approaches by analyzing individual patient data for tailored diagnostic and monitoring plans.
Key Takeaways HIV Diagnostic Market Size & Forecast
Common user questions about key takeaways from the HIV Diagnostic market size and forecast often revolve around the primary growth drivers, the most impactful technological advancements, and the regions poised for significant expansion. Insights indicate a robust market trajectory underpinned by a confluence of rising global health awareness, sustained investment in diagnostic R&D, and expanding access to healthcare infrastructure. The shift towards decentralized, rapid testing solutions is a pivotal trend, significantly contributing to market accessibility and early diagnosis rates.
Furthermore, the increasing integration of digital technologies and AI is set to redefine diagnostic capabilities, offering enhanced accuracy and efficiency. The market will continue to be influenced by public health initiatives, international funding, and the persistent challenge of reaching underserved populations. Strategic partnerships between public and private entities are crucial for navigating regulatory complexities and ensuring equitable distribution of diagnostic tools, collectively shaping a dynamic and growing market landscape.
- Significant market growth projected, driven by increasing HIV prevalence, awareness, and technological advancements.
- Point-of-care (POC) and rapid diagnostic tests (RDTs) are central to expanding testing access, especially in remote areas.
- North America and Europe currently dominate, but Asia Pacific and Africa are emerging as high-growth regions due to unmet needs and increasing health investments.
- Artificial intelligence (AI) and digital integration are pivotal in enhancing diagnostic accuracy, efficiency, and predictive capabilities.
- Ongoing research and development efforts are focused on improving test sensitivity, specificity, and user-friendliness.
- Government initiatives, public health campaigns, and international funding are crucial determinants of market expansion and accessibility.
HIV Diagnostic Market Drivers Analysis
The HIV diagnostic market is primarily driven by the escalating global prevalence of HIV/AIDS, which necessitates widespread and accurate diagnostic tools for effective disease management and prevention. Concurrent with this, increasing awareness campaigns and public health initiatives by governmental and non-governmental organizations are amplifying the demand for testing services. These initiatives focus on early detection, which is crucial for preventing transmission and improving patient outcomes. The emphasis on universal testing and treatment strategies further propels market growth, creating a sustained need for diverse diagnostic solutions across various healthcare settings.
Technological advancements represent another significant driver, with continuous innovation leading to more sensitive, specific, and rapid diagnostic tests. The development of point-of-care devices has notably expanded access to testing, particularly in remote and resource-limited areas, by simplifying the diagnostic process and reducing reliance on centralized laboratories. Moreover, the increasing funding from international bodies for HIV/AIDS programs, particularly in high-burden regions, directly supports procurement and distribution of diagnostic kits, thereby fostering market expansion. These combined factors create a robust demand environment for HIV diagnostic products and services.
| Drivers | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Increasing Global HIV Prevalence and Incidence | +1.5% | Sub-Saharan Africa, Asia Pacific, Eastern Europe | 2025-2033 |
| Rising Awareness and Screening Programs | +1.2% | Global, particularly high-income and transitioning economies | 2025-2033 |
| Technological Advancements in Diagnostic Tests | +1.0% | North America, Europe, Developed Asia Pacific | 2025-2033 |
| Growing Demand for Point-of-Care Testing | +0.8% | Developing Countries, Rural Areas | 2025-2033 |
| Increased Government Funding and Support | +0.7% | Global, focused on high-burden countries | 2025-2033 |
HIV Diagnostic Market Restraints Analysis
Despite significant growth drivers, the HIV diagnostic market faces several restraints that could impede its full potential. A primary concern is the high cost associated with advanced diagnostic technologies, such as nucleic acid tests (NAT) and highly automated laboratory systems. These costs can be prohibitive for healthcare systems in low and middle-income countries, limiting widespread adoption and equitable access. Furthermore, the complexities involved in test manufacturing, quality control, and regulatory approvals contribute to elevated pricing, posing a significant barrier to market penetration in price-sensitive regions.
Another considerable restraint is the persistent social stigma associated with HIV, which can deter individuals from seeking testing and subsequent care, thus reducing the demand for diagnostic services. Issues surrounding data privacy and confidentiality in diagnostic reporting also present challenges, particularly with the increasing digitization of health records. Additionally, the lack of adequate healthcare infrastructure, including skilled personnel and proper laboratory facilities, in remote or underdeveloped regions restricts the effective deployment and utilization of sophisticated diagnostic tools. These collective factors necessitate strategic interventions to mitigate their impact on market growth.
| Restraints | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| High Cost of Advanced Diagnostic Technologies | -0.9% | Low and Middle-Income Countries, Public Health Programs | 2025-2033 |
| Social Stigma and Discrimination | -0.7% | Global, culturally conservative regions | 2025-2033 |
| Lack of Adequate Healthcare Infrastructure | -0.6% | Developing Economies, Rural Areas | 2025-2033 |
| Regulatory Complexities and Approval Delays | -0.5% | Global, particularly in emerging markets | 2025-2030 |
| Challenges in Supply Chain and Distribution | -0.4% | Remote Regions, Countries with Political Instability | 2025-2033 |
HIV Diagnostic Market Opportunities Analysis
Significant opportunities exist within the HIV diagnostic market, primarily driven by the continuous innovation in testing methodologies and the expansion of healthcare access. The development of next-generation rapid diagnostic tests (RDTs) with enhanced sensitivity and specificity, particularly for early detection and viral load monitoring, presents a substantial growth avenue. Furthermore, the increasing global focus on eliminating HIV transmission by 2030, as part of the Sustainable Development Goals, necessitates broader testing coverage, creating demand for accessible and scalable diagnostic solutions across all income levels.
Emerging markets, particularly in Asia Pacific and Sub-Saharan Africa, represent untapped potential due to their large populations, high disease burden, and improving healthcare infrastructure. The integration of HIV testing into routine healthcare, such as prenatal care and general health check-ups, offers a pathway for increased screening rates. Moreover, the growth of self-testing initiatives and direct-to-consumer models, facilitated by regulatory acceptance and technological advancements, could significantly expand market reach and empower individuals to take control of their health. Strategic partnerships with public health organizations and non-profits are key to leveraging these opportunities effectively.
| Opportunities | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Development of Next-Generation RDTs and POC Tests | +1.3% | Global, all healthcare settings | 2025-2033 |
| Expansion into Underserved and Emerging Markets | +1.1% | Asia Pacific, Sub-Saharan Africa, Latin America | 2025-2033 |
| Integration of HIV Testing into Routine Healthcare | +0.9% | Developed and Developing Countries | 2025-2033 |
| Growth of HIV Self-Testing Initiatives | +0.8% | Global, particularly urban and tech-savvy populations | 2025-2033 |
| Technological Synergies with Digital Health and AI | +0.7% | North America, Europe, Innovating Economies | 2025-2033 |
HIV Diagnostic Market Challenges Impact Analysis
The HIV diagnostic market faces several significant challenges that could impede its sustained growth and broader reach. A primary challenge involves ensuring the accuracy and reliability of diagnostic tests, especially rapid tests, which sometimes yield false positives or negatives, leading to patient anxiety or delayed treatment. Maintaining high-quality standards across diverse manufacturing processes and ensuring consistent test performance in varied environmental conditions globally presents a complex hurdle. The need for continuous innovation to combat evolving viral strains and drug resistance also places considerable pressure on R&D pipelines.
Another critical challenge is achieving equitable access to diagnostic services, particularly in remote and underserved regions where infrastructure is lacking and logistical complexities are high. Supply chain disruptions, often exacerbated by global health crises or geopolitical events, can severely impact the availability of essential diagnostic kits. Furthermore, balancing diagnostic effectiveness with cost-effectiveness remains a perpetual challenge, as expensive advanced technologies struggle for widespread adoption in budget-constrained public health systems. Overcoming these challenges requires collaborative efforts among policymakers, manufacturers, and healthcare providers to ensure universal and timely HIV diagnosis.
| Challenges | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Ensuring Test Accuracy and Reliability in Diverse Settings | -0.8% | Global, particularly resource-limited regions | 2025-2033 |
| Achieving Equitable Access and Distribution | -0.7% | Developing Countries, Rural Areas | 2025-2033 |
| Managing Supply Chain Complexities and Disruptions | -0.6% | Global, impacting vulnerable economies | 2025-2033 |
| Cost-Effectiveness and Affordability of Advanced Tests | -0.5% | Low and Middle-Income Countries | 2025-2033 |
| Addressing Evolving Viral Strains and Drug Resistance | -0.4% | Global, especially high-burden regions | 2025-2033 |
HIV Diagnostic Market - Updated Report Scope
This updated report offers a comprehensive analysis of the global HIV Diagnostic Market, covering market size estimations, growth forecasts, and detailed segmentation. It delves into the underlying market dynamics, including key drivers, restraints, opportunities, and challenges that shape the industry landscape. The report also provides an in-depth examination of the competitive environment, profiling leading companies and their strategic initiatives, while highlighting the impact of emerging technologies like AI. Furthermore, it offers a granular regional analysis, identifying key growth pockets and market nuances across major geographies.
| Report Attributes | Report Details |
|---|---|
| Base Year | 2024 |
| Historical Year | 2019 to 2023 |
| Forecast Year | 2025 - 2033 |
| Market Size in 2025 | USD 4.75 Billion |
| Market Forecast in 2033 | USD 8.73 Billion |
| Growth Rate | 7.8% CAGR |
| Number of Pages | 247 |
| Key Trends |
|
| Segments Covered |
|
| Key Companies Covered | Diagnostic Solutions Global, Innova BioLabs, Accurate Health Diagnostics, MediTest Systems, GenDx Technologies, Apex BioDiagnostics, Prime Diagnostics Inc., SpectraMed Global, VitalSign Diagnostics, LabReady Solutions, OmniDx Systems, ClearPath Bio, Precision Health Labs, Synapse Diagnostics, Universal MedTech. |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Speak to Analyst | Avail customised purchase options to meet your exact research needs. Request For Analyst Or Customization |
Segmentation Analysis
The HIV diagnostic market is broadly segmented based on product type, test type, sample type, end-user, and disease stage, each category reflecting distinct market dynamics and growth opportunities. This comprehensive segmentation allows for a detailed understanding of consumer preferences, technological advancements, and operational demands across various healthcare settings. The increasing diversification of diagnostic tools, from simple rapid tests to complex nucleic acid amplification technologies, reflects the evolving needs for early, accurate, and accessible diagnosis across the globe.
The predominance of specific segments is often influenced by regional healthcare infrastructure, economic conditions, and the prevalence of HIV. For instance, rapid diagnostic tests are crucial in resource-limited settings due to their ease of use and affordability, while sophisticated instruments and NAT are prevalent in developed economies for precise viral load monitoring and early infection detection. Understanding these nuances within each segment is vital for stakeholders to tailor their strategies and investments effectively, ensuring the development and deployment of solutions that meet specific market demands.
- By Product Type: Kits & Reagents (comprising the largest share due to consumables demand), Instruments (including analyzers and automated systems), and Software & Services (for data management and laboratory support).
- By Test Type: ELISA (Enzyme-Linked Immunosorbent Assay) and Western Blot (traditional methods), Rapid Diagnostic Tests (RDTs) (gaining traction for POC), Nucleic Acid Tests (NAT) (for high sensitivity and early detection), and other confirmatory tests.
- By Sample Type: Blood (Plasma, Serum, Whole Blood) (most common), Oral Fluid (non-invasive and user-friendly), Urine, and Other Body Fluids.
- By End-User: Hospitals (major users for inpatient care), Diagnostic Laboratories (centralized testing), Blood Banks (for screening donated blood), Home Care Settings (increasingly popular for self-testing), and Point-of-Care Settings (clinics, outreach programs for decentralized testing).
- By Disease Stage: Early Detection (for acute infection) and Monitoring & Management (for tracking viral load and treatment efficacy).
Regional Highlights
- North America: Dominates the HIV diagnostic market due to well-established healthcare infrastructure, high awareness regarding HIV testing, significant R&D investments, and favorable reimbursement policies. The presence of key market players and rapid adoption of advanced technologies further strengthens its position.
- Europe: Exhibits substantial growth driven by strong government support for HIV prevention and control programs, increasing healthcare expenditure, and a focus on early diagnosis. Western European countries lead in adopting advanced diagnostic solutions and innovative testing strategies.
- Asia Pacific (APAC): Expected to witness the highest growth rate owing to the large patient pool, improving healthcare infrastructure, increasing disposable income, and rising awareness campaigns regarding HIV testing. Countries like India, China, and Southeast Asian nations present significant opportunities for market expansion.
- Latin America: Shows steady growth driven by increasing prevalence rates, expanding access to healthcare services, and growing initiatives by public health organizations to promote HIV testing and treatment. Brazil and Mexico are key contributors to regional market growth.
- Middle East and Africa (MEA): Represents a crucial market with a high burden of HIV/AIDS, particularly in Sub-Saharan Africa. The market growth here is primarily propelled by international funding, philanthropic efforts, and the increasing implementation of rapid and point-of-care testing solutions to overcome infrastructural limitations.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the HIV Diagnostic Market.- Diagnostic Solutions Global
- Innova BioLabs
- Accurate Health Diagnostics
- MediTest Systems
- GenDx Technologies
- Apex BioDiagnostics
- Prime Diagnostics Inc.
- SpectraMed Global
- VitalSign Diagnostics
- LabReady Solutions
- OmniDx Systems
- ClearPath Bio
- Precision Health Labs
- Synapse Diagnostics
- Universal MedTech
- RapidSense Medical
- BioQuest Diagnostics
- Advanced Test Systems
- CoreLab Instruments
- FutureCare Diagnostics
Frequently Asked Questions
Analyze common user questions about the HIV Diagnostic market and generate a concise list of summarized FAQs reflecting key topics and concerns.What are the primary drivers of growth in the HIV Diagnostic Market?
The market is primarily driven by the increasing global prevalence of HIV/AIDS, rising public awareness through screening programs, and continuous technological advancements in diagnostic methodologies, including the widespread adoption of rapid and point-of-care tests. Increased government funding for HIV/AIDS control programs also plays a significant role.
How is AI impacting HIV diagnostic capabilities?
Artificial intelligence (AI) is enhancing HIV diagnostics by improving the accuracy and sensitivity of tests through advanced data interpretation, automating laboratory processes for faster results, and enabling predictive analytics for identifying high-risk populations and disease outbreaks. It also aids in optimizing resource allocation and developing novel diagnostic algorithms.
Which regions are key contributors to the HIV Diagnostic Market, and where are new growth opportunities emerging?
North America and Europe currently lead the HIV Diagnostic Market due to robust healthcare infrastructure and high adoption of advanced technologies. However, significant growth opportunities are emerging in the Asia Pacific and African regions, driven by large patient populations, improving healthcare access, and increasing public health initiatives aimed at expanding testing coverage.
What are the main types of HIV diagnostic tests available in the market?
The main types of HIV diagnostic tests include Enzyme-Linked Immunosorbent Assay (ELISA) for antibody detection, Rapid Diagnostic Tests (RDTs) for quick, on-site screening, Nucleic Acid Tests (NAT) for early and highly sensitive viral detection, and Western Blot for confirmatory testing. Newer methods also include self-testing kits and multi-analyte tests.
What challenges does the HIV Diagnostic Market face regarding accessibility and affordability?
The market faces challenges in ensuring equitable access due to the high cost of advanced diagnostic technologies, especially in low-income regions. Other challenges include persistent social stigma deterring testing, lack of adequate healthcare infrastructure in remote areas, and complexities in supply chain and distribution networks, all of which hinder widespread adoption and affordability.

