Eribulin Mesylate API Market
Eribulin Mesylate API Market Size, Scope, Growth, Trends and By Segmentation Types, Applications, Regional Analysis and Industry Forecast (2025-2033)
Report ID : RI_701143 | Last Updated : July 29, 2025 |
Format : ![]()
![]()
![]()
![]()
Eribulin Mesylate API Market Size
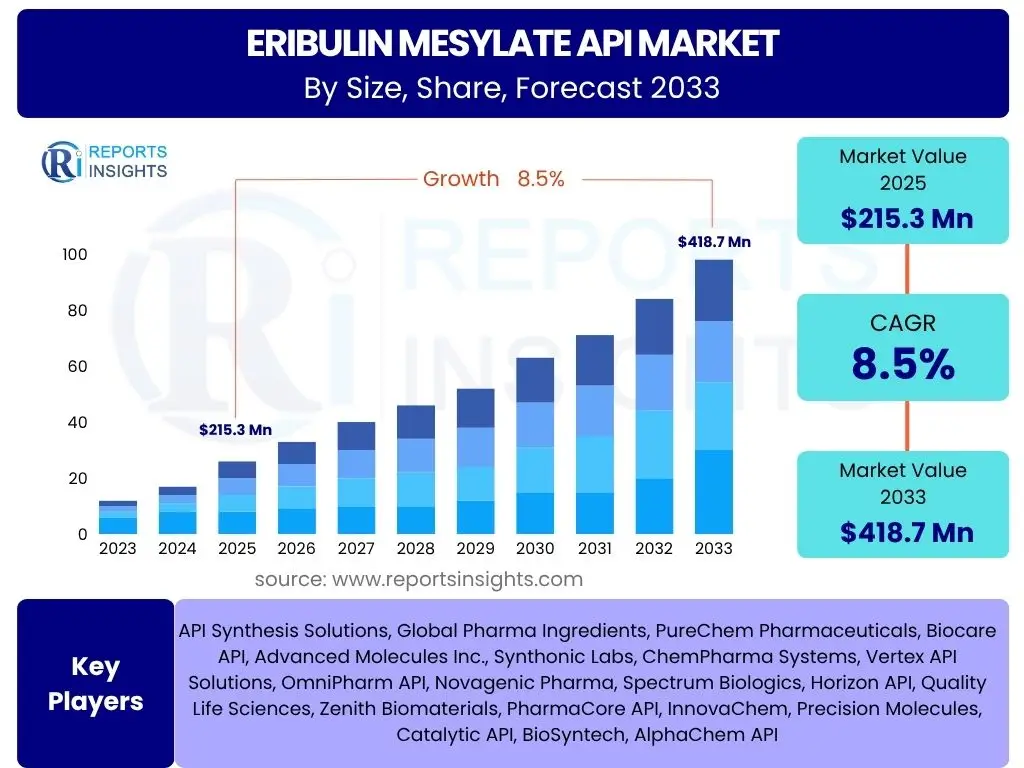
According to Reports Insights Consulting Pvt Ltd, The Eribulin Mesylate API Market is projected to grow at a Compound Annual Growth Rate (CAGR) of 8.5% between 2025 and 2033. The market is estimated at USD 215.3 million in 2025 and is projected to reach USD 418.7 million by the end of the forecast period in 2033.
Key Eribulin Mesylate API Market Trends & Insights
The Eribulin Mesylate API market is currently undergoing significant transformation, driven by advancements in oncology research and pharmaceutical manufacturing. A prominent trend involves the increasing demand for high-purity API, essential for enhancing drug efficacy and reducing adverse effects in patients. This push for purity is coupled with stringent regulatory requirements globally, compelling manufacturers to invest in advanced synthesis and purification technologies to meet the evolving quality standards.
Another key insight is the growing emphasis on sustainable and cost-effective manufacturing processes. Companies are exploring innovative chemical synthesis routes and biocatalytic approaches to reduce the environmental footprint and optimize production costs without compromising quality. Furthermore, the expansion of clinical trials for Eribulin Mesylate into new indications or combination therapies is creating sustained demand for the API, indicating a broadening application landscape beyond its current approved uses in breast cancer and liposarcoma.
The market also observes a trend towards strategic collaborations between API manufacturers and pharmaceutical companies. These partnerships aim to ensure a stable supply chain, facilitate technology transfer, and accelerate the development of new drug formulations. Such collaborations are crucial for navigating the complex regulatory landscape and for bringing new therapeutic options to market efficiently, thus fostering market growth and innovation.
- Escalating demand for high-purity Eribulin Mesylate API.
- Development of sustainable and cost-efficient manufacturing processes.
- Expansion of Eribulin Mesylate clinical trials into new indications.
- Increased strategic collaborations between API producers and drug developers.
- Stricter global regulatory standards for pharmaceutical ingredients.
AI Impact Analysis on Eribulin Mesylate API
The integration of Artificial Intelligence (AI) and machine learning (ML) technologies is poised to significantly transform various facets of the Eribulin Mesylate API market, from drug discovery and synthesis to supply chain management. Users frequently inquire about AI's potential to accelerate the identification of novel synthesis pathways, optimize reaction conditions for higher yields and purity, and predict potential impurities, thereby streamlining the manufacturing process and reducing development timelines. AI-driven retrosynthesis tools, for instance, can analyze complex molecular structures and propose efficient synthetic routes, potentially reducing the need for extensive trial-and-error experimentation.
Furthermore, AI is expected to enhance quality control and assurance processes within API manufacturing. Predictive analytics, powered by AI, can monitor real-time production data to identify deviations or potential quality issues before they escalate, ensuring consistent product quality and reducing batch failures. This proactive approach not only saves significant resources but also enhances compliance with rigorous regulatory guidelines. The ability of AI to analyze vast datasets related to chemical properties and process parameters allows for unprecedented precision and control in API production.
Beyond the lab, AI's impact extends to supply chain optimization for Eribulin Mesylate API. AI algorithms can forecast demand more accurately, optimize inventory levels, and identify potential disruptions in the supply chain, such as raw material shortages or logistical challenges. This leads to more resilient and efficient supply networks, ensuring a steady and reliable supply of Eribulin Mesylate API to pharmaceutical companies globally. The strategic deployment of AI tools is therefore anticipated to drive both operational efficiency and market competitiveness.
- Optimization of Eribulin Mesylate API synthesis pathways and reaction conditions.
- Enhanced quality control and impurity detection through AI-driven analytics.
- Improved supply chain forecasting and resilience for API distribution.
- Acceleration of drug discovery and development related to Eribulin derivatives.
- Facilitation of real-time process monitoring and predictive maintenance in manufacturing.
Key Takeaways Eribulin Mesylate API Market Size & Forecast
Analysis of user inquiries about the Eribulin Mesylate API market size and forecast reveals a strong interest in understanding the primary growth drivers and the long-term sustainability of demand. A key takeaway is that the market is underpinned by the persistent global burden of various cancers, particularly metastatic breast cancer and liposarcoma, for which Eribulin Mesylate remains a vital therapeutic option. This sustained clinical demand ensures a foundational growth trajectory for the API market, supported by ongoing research into its expanded applications.
Another significant insight is the critical role of innovation in manufacturing and purity standards. The market's future growth is heavily reliant on API manufacturers' ability to produce Eribulin Mesylate that meets increasingly stringent regulatory and quality requirements, while also achieving cost efficiencies. Investment in advanced purification techniques and optimized synthesis processes will be paramount for companies aiming to capture a larger market share and sustain competitiveness.
The projected growth trajectory, indicated by the Compound Annual Growth Rate (CAGR) and market value forecasts, highlights a robust expansion over the next decade. This growth is anticipated to be fueled by a combination of factors, including increasing healthcare expenditure, a rising incidence of target diseases, and the potential for new clinical indications or combination therapies involving Eribulin. Strategic partnerships and a focus on supply chain resilience will be key for stakeholders to capitalize on these opportunities and mitigate potential challenges.
- Consistent demand driven by oncology treatment needs.
- Crucial importance of high-purity API and manufacturing innovation.
- Robust market expansion projected over the forecast period.
- Potential for new clinical applications to drive further growth.
- Strategic partnerships enhancing supply chain stability and market access.
Eribulin Mesylate API Market Drivers Analysis
The Eribulin Mesylate API market is primarily driven by the increasing global incidence of cancers for which Eribulin Mesylate is an approved and effective treatment. Specifically, the rising prevalence of metastatic breast cancer and advanced liposarcoma globally fuels a consistent demand for this active pharmaceutical ingredient. As diagnostic capabilities improve and treatment access expands, particularly in emerging economies, the patient pool eligible for Eribulin-based therapies is expected to grow, directly translating into higher API consumption.
Advancements in oncology research and development also serve as a significant driver. Ongoing clinical trials exploring new indications for Eribulin Mesylate, or its use in combination with other therapeutic agents, could unlock new revenue streams and expand its market reach. Positive outcomes from these trials would broaden the therapeutic scope of Eribulin, leading to an increased requirement for the API. This continuous innovation in therapeutic applications reinforces the market's growth potential.
Furthermore, the growing focus on personalized medicine and targeted therapies contributes to market expansion. As healthcare systems increasingly adopt tailored treatment approaches based on specific patient characteristics and tumor profiles, the demand for highly efficacious and specific drugs like Eribulin Mesylate is amplified. The established efficacy and safety profile of Eribulin Mesylate in its approved indications ensure its continued preference among oncologists, thereby sustaining API demand.
| Drivers | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Rising Cancer Incidence (Breast Cancer, Liposarcoma) | +2.5% | Global, particularly North America, Europe, APAC | 2025-2033 (Long-term) |
| Advancements in Oncology Research & New Indications | +1.8% | North America, Europe, Key Emerging Markets | 2026-2033 (Mid to Long-term) |
| Increasing Healthcare Expenditure & Access to Treatment | +1.5% | APAC, Latin America, Middle East, Africa | 2025-2033 (Long-term) |
| Growing Adoption of Targeted Therapies | +1.2% | Global, especially developed regions | 2025-2030 (Mid-term) |
| Development of Cost-Effective Synthesis Technologies | +1.0% | Global, particularly India, China | 2027-2033 (Long-term) |
Eribulin Mesylate API Market Restraints Analysis
The Eribulin Mesylate API market faces several significant restraints that could impede its growth trajectory. One primary challenge is the high cost associated with the research, development, and manufacturing of Eribulin Mesylate. The complexity of its chemical synthesis requires specialized equipment and highly skilled personnel, leading to elevated production costs which can, in turn, affect the affordability and accessibility of the final drug product, particularly in price-sensitive markets.
Another crucial restraint stems from the stringent regulatory landscape and the extensive approval processes for pharmaceutical APIs. Compliance with Good Manufacturing Practices (GMP) and other international quality standards demands significant investment in infrastructure, quality control systems, and regulatory filings. Any delays or failures in meeting these rigorous requirements can postpone market entry or lead to product recalls, negatively impacting market growth and manufacturer confidence.
Furthermore, the availability of alternative treatment options and the emergence of biosimilars or generic versions of Eribulin Mesylate could pose a competitive threat. As patents expire or new, more cost-effective therapies enter the market, the demand for proprietary Eribulin Mesylate API might face downward pressure. This competitive intensity necessitates continuous innovation and differentiation for API manufacturers to maintain their market position.
| Restraints | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| High Cost of API Manufacturing | -1.5% | Global, particularly developing markets | 2025-2033 (Long-term) |
| Stringent Regulatory Requirements & Compliance | -1.3% | North America, Europe, Japan | 2025-2030 (Mid-term) |
| Competition from Alternative Therapies & Generics | -1.0% | Global | 2028-2033 (Long-term) |
| Supply Chain Volatility and Raw Material Availability | -0.8% | Global, particularly Asia Pacific | 2025-2028 (Short to Mid-term) |
Eribulin Mesylate API Market Opportunities Analysis
Significant opportunities exist within the Eribulin Mesylate API market, particularly through the expansion into emerging economies. Countries in Asia Pacific, Latin America, and the Middle East and Africa are witnessing a rapid improvement in healthcare infrastructure, increased healthcare expenditure, and a growing awareness of advanced cancer treatments. This demographic shift and economic development present a vast untapped market for Eribulin-based therapies, thus creating substantial demand for its API.
The development of new drug delivery systems and formulations for Eribulin Mesylate also presents a promising avenue for market growth. Innovations such as oral formulations, extended-release versions, or more targeted delivery mechanisms could improve patient compliance, enhance therapeutic outcomes, and expand the drug's applicability. API manufacturers that can support these novel formulations through specialized production capabilities will find new market niches.
Furthermore, strategic partnerships and contract manufacturing agreements offer considerable opportunities for API producers. As pharmaceutical companies increasingly outsource their API manufacturing to focus on core competencies like R&D and marketing, there is a growing demand for reliable and high-quality contract development and manufacturing organizations (CDMOs). These collaborations can provide API manufacturers with stable revenue streams and access to advanced technologies and broader market intelligence.
| Opportunities | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| Expansion into Emerging Markets | +2.0% | APAC, Latin America, MEA | 2025-2033 (Long-term) |
| Development of New Drug Formulations & Delivery Systems | +1.5% | Global | 2027-2033 (Long-term) |
| Strategic CDMO Partnerships & Outsourcing Trends | +1.3% | Global | 2025-2030 (Mid-term) |
| Technological Advancements in Synthesis & Purification | +1.0% | Global | 2026-2033 (Long-term) |
Eribulin Mesylate API Market Challenges Impact Analysis
The Eribulin Mesylate API market faces several inherent challenges that demand strategic navigation from stakeholders. One significant challenge is the complexity and sensitivity of the Eribulin Mesylate molecule, which makes its synthesis and purification highly intricate. Maintaining consistent purity and quality throughout the manufacturing process, particularly at commercial scale, requires substantial expertise and specialized infrastructure. Any deviations can lead to batch rejections and significant financial losses, impacting supply chain reliability.
Another major challenge is the intense competition among API manufacturers, coupled with pricing pressures from pharmaceutical companies. As more players enter the market or existing ones optimize their processes, there is a constant downward pressure on API prices. This competitive environment necessitates continuous innovation in manufacturing processes to achieve cost efficiencies without compromising product quality, posing a dilemma for manufacturers trying to maintain profitability.
Furthermore, disruptions in the global supply chain, including raw material sourcing, logistics, and geopolitical instabilities, can significantly impact the production and timely delivery of Eribulin Mesylate API. Dependency on a limited number of specialized raw material suppliers or specific geographical regions for key intermediates makes the supply chain vulnerable to external shocks. Ensuring a robust and diversified supply network remains a critical challenge for sustained market operations.
| Challenges | (~) Impact on CAGR % Forecast | Regional/Country Relevance | Impact Time Period |
|---|---|---|---|
| High Complexity of Synthesis & Purity Requirements | -1.2% | Global | 2025-2033 (Long-term) |
| Intense Price Competition & Profitability Pressures | -1.0% | Global | 2025-2030 (Mid-term) |
| Supply Chain Disruptions & Raw Material Volatility | -0.9% | Global, especially APAC | 2025-2028 (Short to Mid-term) |
| Intellectual Property & Patent Protection Issues | -0.7% | Global | 2028-2033 (Long-term) |
Eribulin Mesylate API Market - Updated Report Scope
This comprehensive market research report delves into the intricate dynamics of the Eribulin Mesylate API market, offering an in-depth analysis of its current landscape and future growth projections. It covers detailed segmentation, regional performance, competitive analysis, and an evaluation of critical market drivers, restraints, opportunities, and challenges influencing the industry from 2019 to 2033. The report is designed to provide actionable insights for stakeholders, aiding strategic decision-making in this vital pharmaceutical sector.
| Report Attributes | Report Details |
|---|---|
| Base Year | 2024 |
| Historical Year | 2019 to 2023 |
| Forecast Year | 2025 - 2033 |
| Market Size in 2025 | USD 215.3 million |
| Market Forecast in 2033 | USD 418.7 million |
| Growth Rate | 8.5% |
| Number of Pages | 257 |
| Key Trends |
|
| Segments Covered |
|
| Key Companies Covered | API Synthesis Solutions, Global Pharma Ingredients, PureChem Pharmaceuticals, Biocare API, Advanced Molecules Inc., Synthonic Labs, ChemPharma Systems, Vertex API Solutions, OmniPharm API, Novagenic Pharma, Spectrum Biologics, Horizon API, Quality Life Sciences, Zenith Biomaterials, PharmaCore API, InnovaChem, Precision Molecules, Catalytic API, BioSyntech, AlphaChem API |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Speak to Analyst | Avail customised purchase options to meet your exact research needs. Request For Analyst Or Customization |
Segmentation Analysis
The Eribulin Mesylate API market is comprehensively segmented to provide a granular understanding of its various facets and their contribution to overall market dynamics. This segmentation facilitates a detailed analysis of specific product types, application areas, end-use industries, manufacturing processes, and forms, allowing for targeted strategic planning and investment. Each segment reflects unique demand patterns, technological requirements, and competitive landscapes.
The "By Type" segment differentiates between High Purity and Standard Grade Eribulin Mesylate API, highlighting the increasing industry emphasis on superior quality for enhanced therapeutic outcomes. The "By Application" segment showcases the primary therapeutic areas, with breast cancer and liposarcoma treatment being the dominant categories, along with emerging "Other Oncology Applications" that could drive future growth. Understanding these applications is crucial for anticipating demand shifts.
Further segmentation by "End-Use Industry" provides insights into the primary consumers of Eribulin Mesylate API, including pharmaceutical companies engaged in drug manufacturing, research and academic institutions exploring new uses, and contract development and manufacturing organizations (CDMOs) playing a pivotal role in outsourcing trends. The "By Manufacturing Process" segment details the prevalent synthesis methods, such as chemical synthesis and biocatalytic synthesis, indicating technological evolution. Finally, the "By Form" segment delineates the physical states in which the API is supplied, typically as powder or liquid concentrate, addressing specific formulation needs.
- By Type:
- High Purity Eribulin Mesylate API
- Standard Grade Eribulin Mesylate API
- By Application:
- Breast Cancer Treatment
- Liposarcoma Treatment
- Other Oncology Applications
- By End-Use Industry:
- Pharmaceutical Companies
- Research & Academic Institutions
- Contract Development and Manufacturing Organizations (CDMOs)
- By Manufacturing Process:
- Chemical Synthesis
- Biocatalytic Synthesis
- By Form:
- Powder
- Liquid Concentrate
Regional Highlights
- North America: This region is a dominant market for Eribulin Mesylate API due to its well-established healthcare infrastructure, high healthcare expenditure, significant prevalence of cancer cases, and robust research and development activities in oncology. The presence of major pharmaceutical companies and advanced treatment adoption contribute significantly to market size.
- Europe: Europe represents another substantial market, characterized by stringent regulatory frameworks, a high standard of medical care, and continuous investment in cancer research. Key countries like Germany, France, and the UK are major contributors, driving demand for high-quality Eribulin Mesylate API.
- Asia Pacific (APAC): The APAC region is projected to exhibit the highest growth rate, fueled by improving healthcare access, rising disposable incomes, increasing awareness about advanced cancer therapies, and a large patient pool. Countries like China and India are emerging as key manufacturing hubs and consumption markets due to their growing pharmaceutical industries and expanding patient base.
- Latin America: This region is experiencing steady growth, driven by increasing government investments in healthcare, expanding access to specialized cancer treatments, and the rising burden of chronic diseases. Brazil and Mexico are significant markets within this region.
- Middle East and Africa (MEA): The MEA market is gradually expanding, supported by developing healthcare infrastructure, increasing foreign direct investment in the healthcare sector, and a growing focus on improving cancer care services, particularly in countries like Saudi Arabia and South Africa.
Top Key Players
The market research report includes a detailed profile of leading stakeholders in the Eribulin Mesylate API Market.- API Synthesis Solutions
- Global Pharma Ingredients
- PureChem Pharmaceuticals
- Biocare API
- Advanced Molecules Inc.
- Synthonic Labs
- ChemPharma Systems
- Vertex API Solutions
- OmniPharm API
- Novagenic Pharma
- Spectrum Biologics
- Horizon API
- Quality Life Sciences
- Zenith Biomaterials
- PharmaCore API
- InnovaChem
- Precision Molecules
- Catalytic API
- BioSyntech
- AlphaChem API
Frequently Asked Questions
Analyze common user questions about the Eribulin Mesylate API market and generate a concise list of summarized FAQs reflecting key topics and concerns.What is Eribulin Mesylate API primarily used for?
Eribulin Mesylate API is primarily used as the active pharmaceutical ingredient in drugs for treating metastatic breast cancer and advanced liposarcoma, particularly in patients who have previously received other chemotherapy regimens.
What factors are driving the growth of the Eribulin Mesylate API market?
The market is driven by the increasing global incidence of target cancers, ongoing oncology research leading to new indications, advancements in manufacturing technologies, and growing healthcare expenditure in emerging economies.
How does AI impact the Eribulin Mesylate API manufacturing process?
AI impacts manufacturing by optimizing synthesis pathways, enhancing quality control and impurity detection through predictive analytics, and improving supply chain efficiency and forecasting, leading to more efficient and reliable production.
What are the main challenges faced by Eribulin Mesylate API manufacturers?
Key challenges include the high complexity and cost of synthesis, stringent regulatory compliance, intense price competition, and potential disruptions in the global raw material supply chain.
Which regions are key contributors to the Eribulin Mesylate API market?
North America and Europe are major contributors due to advanced healthcare systems, while Asia Pacific, particularly China and India, is poised for the fastest growth owing to improving healthcare access and expanding pharmaceutical industries.